Question: ur reactions are given below. For the first, predict the products, write the balanced chemical equation, and classify it a precipitation, acid-base, gas-forming, or oxidation-reduction
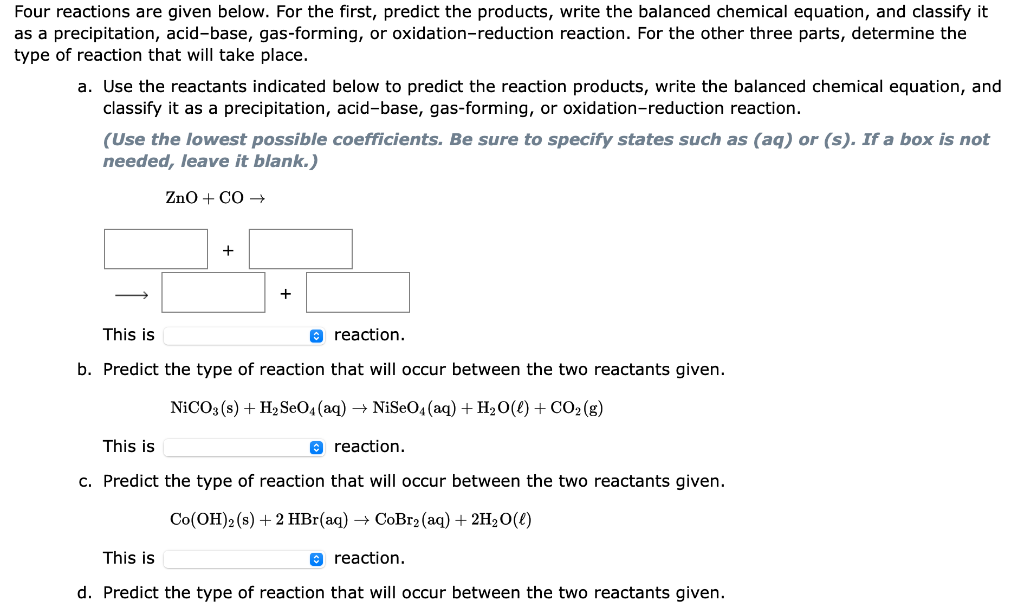


ur reactions are given below. For the first, predict the products, write the balanced chemical equation, and classify it a precipitation, acid-base, gas-forming, or oxidation-reduction reaction. For the other three parts, determine the se of reaction that will take place. a. Use the reactants indicated below to predict the reaction products, write the balanced chemical equation, and classify it as a precipitation, acid-base, gas-forming, or oxidation-reduction reaction. (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) ZnO+CO + + This is reaction. b. Predict the type of reaction that will occur between the two reactants given. NiCO3(s)+H2SeO4(aq)NiSeO4(aq)+H2O()+CO2(g) This is reaction. c. Predict the type of reaction that will occur between the two reactants given. Co(OH)2(s)+2HBr(aq)CoBr2(aq)+2H2O() This is reaction. d. Predict the type of reaction that will occur between the two reactants given. d. Predict the type of reaction that will occur between the two reactants given. AgCH3CO2(aq)+NaCl(aq)AgCl(s)+NaCH3CO2(aq) This is reaction. a precipitation an acid-base a gas-forming an oxidation-reduction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


