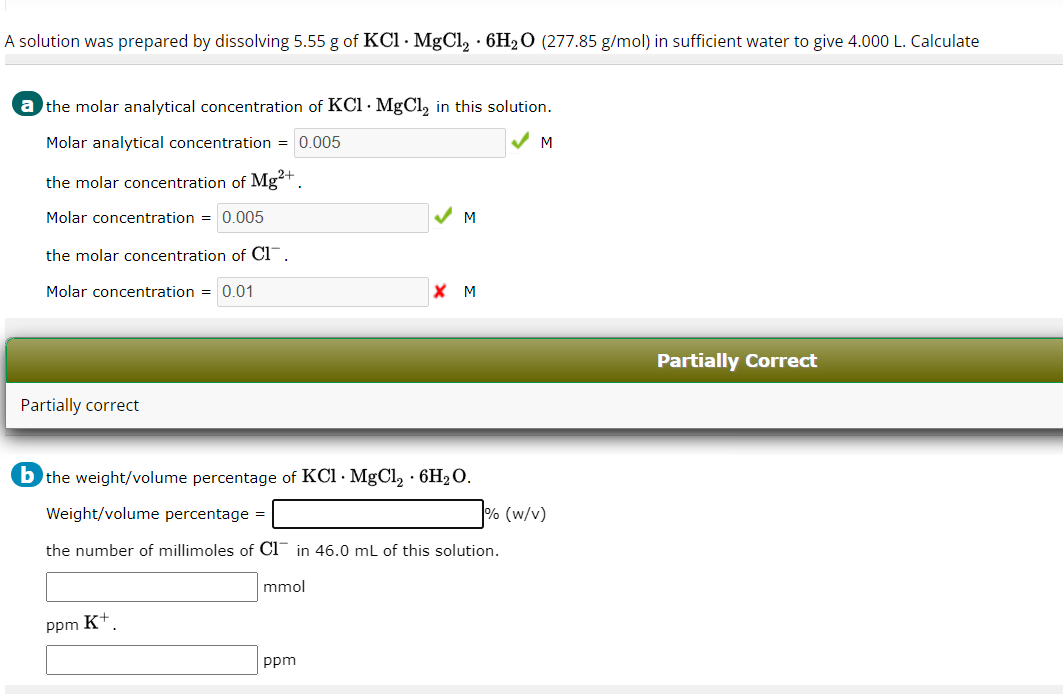
Question: Part B . ( by the way the molar concentration is 0 . 0 1 5 ) . Find the weight volume percentage of KCl
Part Bby the way the molar concentration is Find the weight volume percentage of KClMgClHO Then find the number of millimoles of Cl in ml of this solution. Then find the ppm of K Explain all steps and show work.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


