Question: PC13 has a trigonal pyramidal geometry and has bond lengths of 204.3 pm and Cl-P-Cl angles of 100.1. Calculate the rotational constants , B,
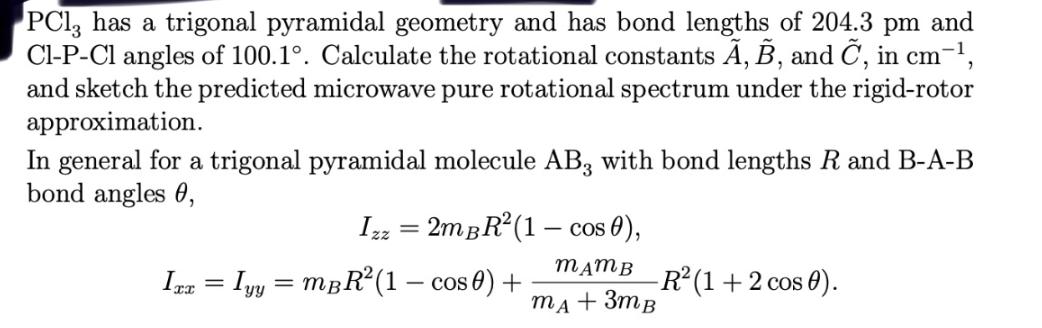
PC13 has a trigonal pyramidal geometry and has bond lengths of 204.3 pm and Cl-P-Cl angles of 100.1. Calculate the rotational constants , B, and C, in cm, and sketch the predicted microwave pure rotational spectrum under the rigid-rotor approximation. In general for a trigonal pyramidal molecule AB, with bond lengths R and B-A-B bond angles 0, Izz=2mBR(1- cos 0), Ixx = Iyy = mBR (1 - cos 0) + MAMB MA + 3MB -R (1 + 2 cos 0).
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


