Question: please answer asap (you may use each option more than once and the options for this question are revealed at the bottom) Match each statement
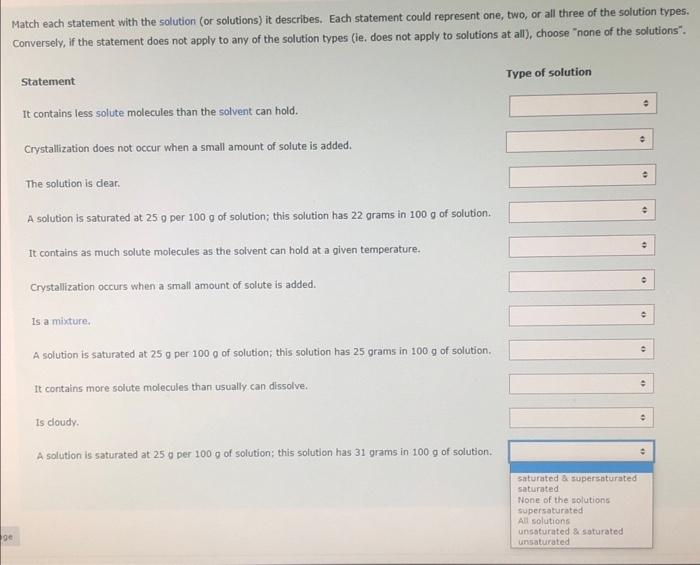
Match each statement with the solution (or solutions) it describes. Each statement could represent one, two, or all three of the solution types. Conversely, if the statement does not apply to any of the solution types (ie. does not apply to solutions at all), choose "none of the solutions". Statement Type of solution It contains less solute molecules than the solvent can hold. Crystallization does not occur when a small amount of solute is added. The solution is dear. A solution is saturated at 25g per 100g of solution; this solution has 22 grams in 100g of solution. It contains as much solute molecules as the solvent can hold at a given temperature. Crystalization occurs when a small amount of solute is added. Is a mixture. A solution is saturated at 25g per 100g of solution; this solution has 25grams in 100g of solution. It contains more solute molecules than usually can dissolve. is cloudy. A solution is saturated at 250 per 100g of solution; this solution has 31 grams in 100g of solution. saturated \& supersaturated saturated None of the solutions supersaturated All solutions unsaturated 2 saturated unsaturated
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


