Question: Please EXPLAIN and solve EACH / ALL part(s) in Question #6 ! DOUBLE CHECK YOUR WORK AND ANSWER(S) . PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS
Please EXPLAIN and solve EACH/ALL part(s) in Question #6!
DOUBLE CHECK YOUR WORK AND ANSWER(S).
PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS, & CALCULATIONS STEP-BY-STEP USING PEN AND PAPER! I AM NEW TO CHEMISTRY! I AM A COMPLETE NEWBIE!
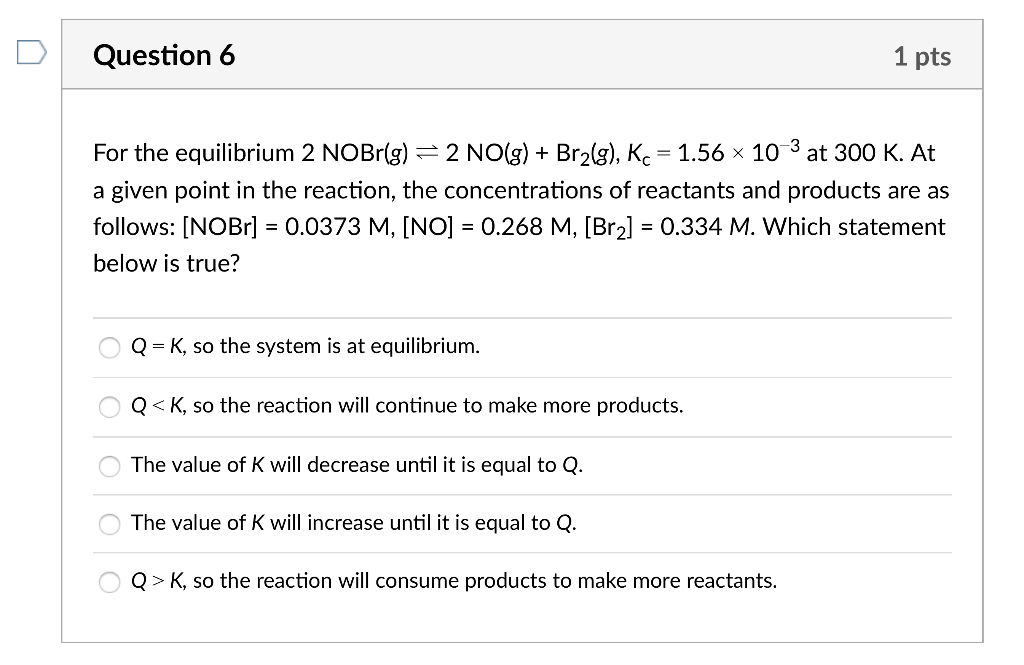
For the equilibrium 2NOBr(g)2NO(g)+Br2(g),Kc=1.56103 at 300K. At a given point in the reaction, the concentrations of reactants and products are as follows: [NOBr]=0.0373M,[NO]=0.268M,[Br2]=0.334M. Which statement below is true? Q=K, so the system is at equilibrium. Q
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


