Question: Please EXPLAIN and solve EACH / ALL part(s) in Question #10 ! DOUBLE CHECK YOUR WORK AND ANSWER(S) . PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS
Please EXPLAIN and solve EACH/ALL part(s) in Question #10!
DOUBLE CHECK YOUR WORK AND ANSWER(S).
PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS, & CALCULATIONS STEP-BY-STEP USING PEN AND PAPER! I AM NEW TO CHEMISTRY! I AM A COMPLETE NEWBIE!
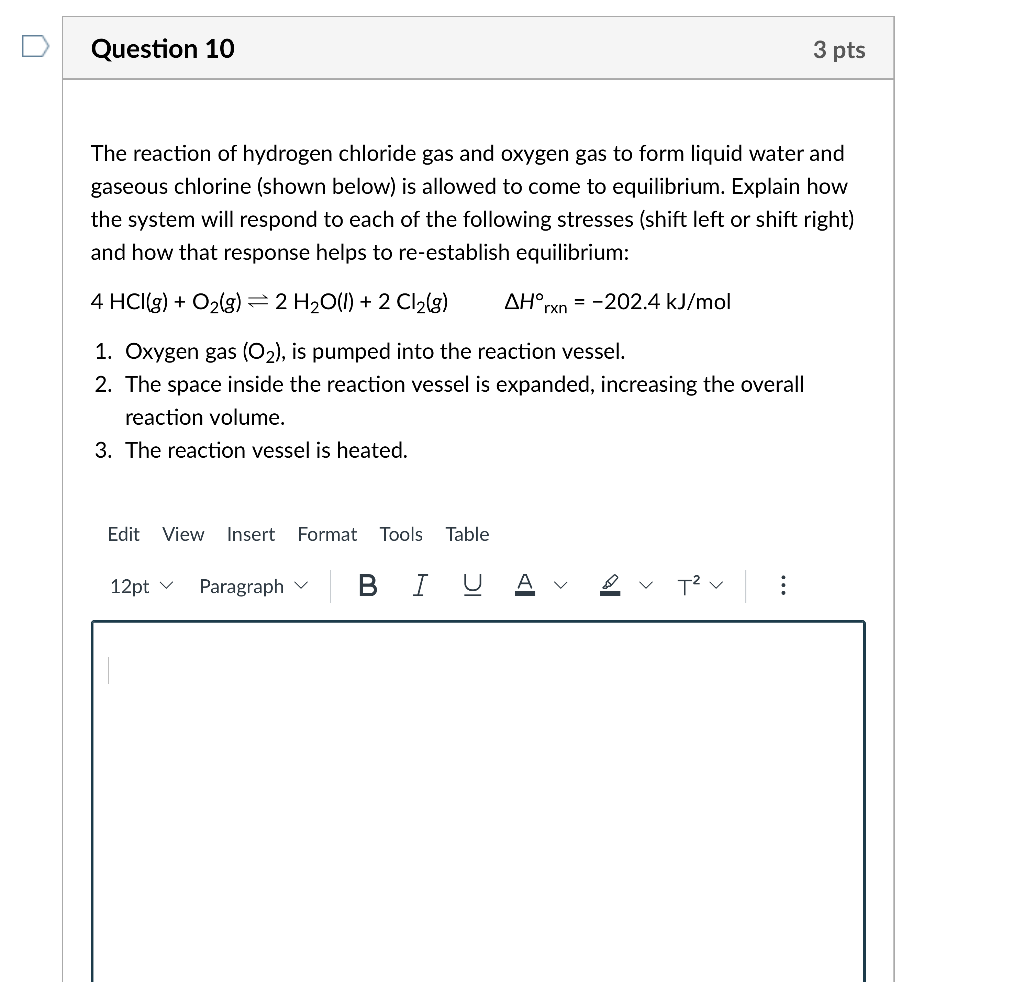
The reaction of hydrogen chloride gas and oxygen gas to form liquid water and gaseous chlorine (shown below) is allowed to come to equilibrium. Explain how the system will respond to each of the following stresses (shift left or shift right) and how that response helps to re-establish equilibrium: 4HCl(g)+O2(g)2H2O(I)+2Cl2(g)Hrxn=202.4kJ/mol 1. Oxygen gas (O2), is pumped into the reaction vessel. 2. The space inside the reaction vessel is expanded, increasing the overall reaction volume. 3. The reaction vessel is heated
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


