Question: Please EXPLAIN and solve EACH / ALL part(s) in Question #1 ! DOUBLE CHECK YOUR WORK AND ANSWER(S) . PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS
Please EXPLAIN and solve EACH/ALL part(s) in Question #1!
DOUBLE CHECK YOUR WORK AND ANSWER(S).
PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS, & CALCULATIONS STEP-BY-STEP USING PEN AND PAPER! I AM NEWTO CHEMISTRY! I AM A COMPLETE NEWBIE!
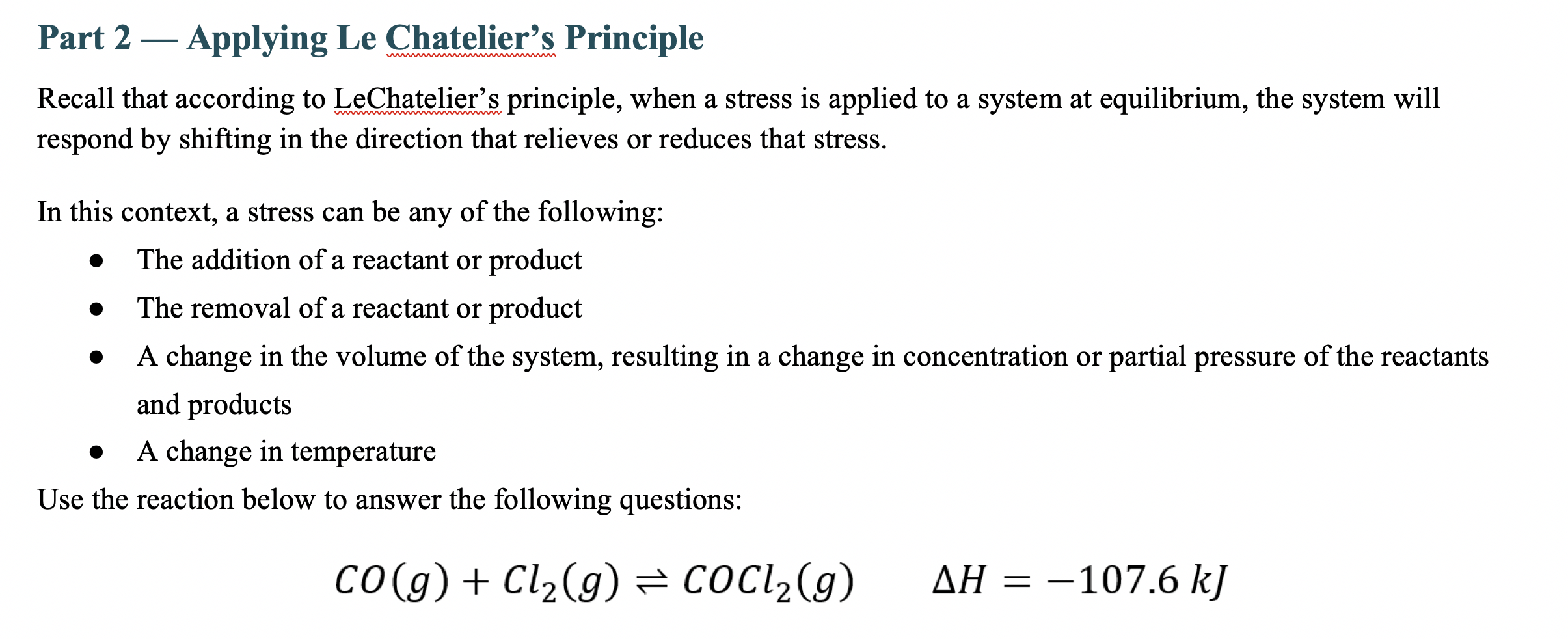


Part 2 - Applying Le Chatelier's Principle Recall that according to LeChatelier's principle, when a stress is applied to a system at equilibrium, the system will respond by shifting in the direction that relieves or reduces that stress. In this context, a stress can be any of the following: - The addition of a reactant or product - The removal of a reactant or product - A change in the volume of the system, resulting in a change in concentration or partial pressure of the reactants and products - A change in temperature Use the reaction below to answer the following questions: CO(g)+C2(g)COCl2(g)H=107.6kJ 1A. How will adding chlorine, Cl2, to the reaction vessel affect the amount of COCl2 formed at equilibrium? Type your answer here. 1B. How will removing carbon monoxide from the reaction vessel affect the amount of COCl2 formed at equilibrium? Type your answer here. 1C. What impact would decreasing the size of the reaction vessel (using a movable piston) have on the amount of COCl2 formed? Type your answer here. 1D. What impact would adding a non-reactive gas (like argon) to the reaction vessel have on the amount of COCl2 formed? Type your answer here. 1E. Will the equilibrium shift if the reaction vessel is heated? If so, which direction and why? 1F. If a catalyst, such as iron (III) oxide, were added to the reaction, what impact would it have on K (the equilibrium constant)? Type your answer here
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


