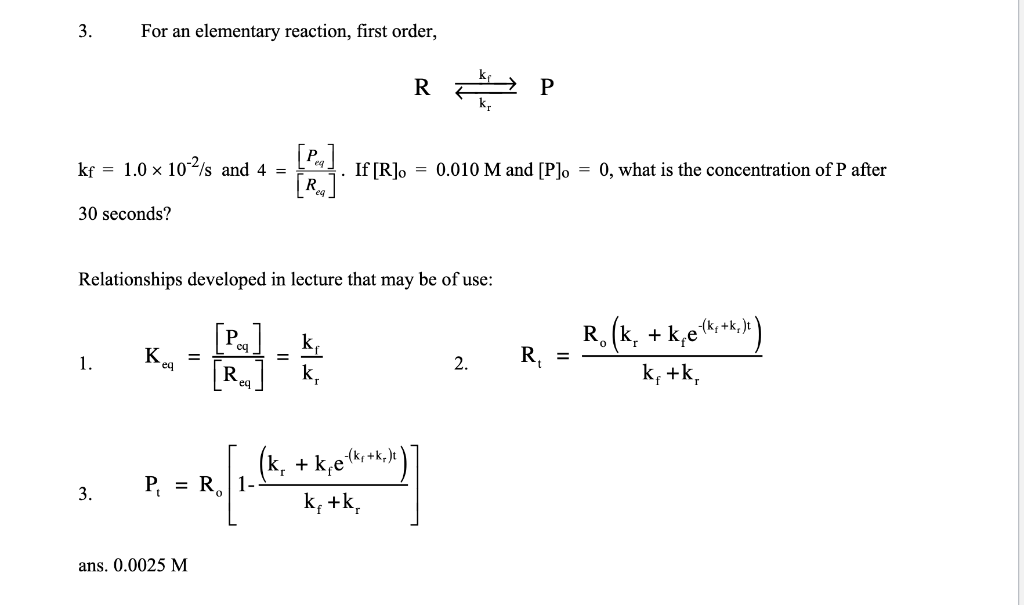
Question: Please help solve and explain the problem the answer is provided 3. For an elementary reaction, first order, RkrkfP kf=1.0102/s and 4=[Req][Peq]. If [R]o=0.010M and
Please help solve and explain the problem the answer is provided
3. For an elementary reaction, first order, RkrkfP kf=1.0102/s and 4=[Req][Peq]. If [R]o=0.010M and [P]o=0, what is the concentration of P after 30 seconds? Relationships developed in lecture that may be of use: 1. Keq=[Req][Peq]=krkf 2. Rt=kf+krRo(kr+kfe(kf+kf)t) 3. Pt=Ro[1kf+kr(kr+kfe(kf+kr)t)] ans. 0.0025M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


