Question: please help What is the molecular geometry and electron domain geometry respectively for ammonia, NH3, below? H-N-H I- Trigonal Pyramidal and Tetrahedral wb Tetrahedral and
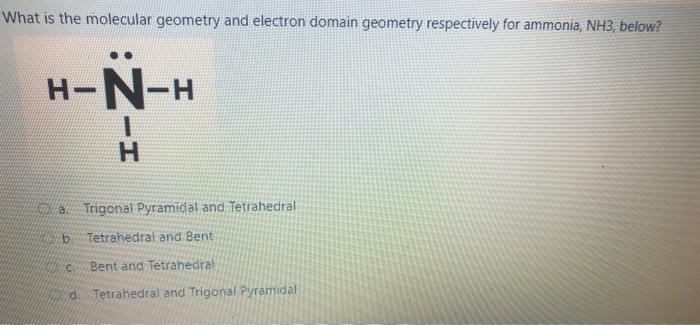


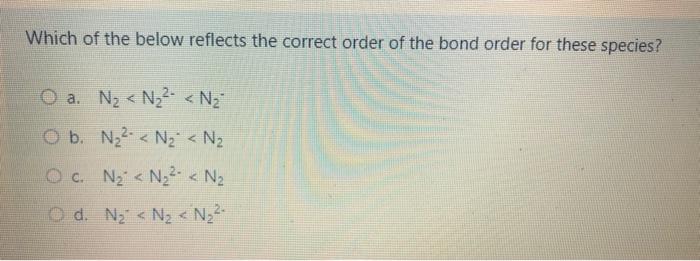

What is the molecular geometry and electron domain geometry respectively for ammonia, NH3, below? H-N-H I- Trigonal Pyramidal and Tetrahedral wb Tetrahedral and Bent Bent and Tetrahedral d. Tetrahedral and Trigonal Pyramidal Which statement is false about bond order? a. The greater the bond order the greater the bond length b. Bond order takes into consideration both bonding and anti-bonding electrons The greater the bond order the greater the number of bonds present. ed. The greater the bond order the more stable the molecule. Which of the below is false regarding covalent interactions? O a. Heteronuclear interactions are interactions between atoms of different elements O b. Heteronuclear bonds tend to be non-polar. Oc Homonuclear interactions are characterized by equal sharing of electron density. d. Homonuclear interactions are interactions between atoms of the same element. Which of the below reflects the correct order of the bond order for these species? O a. Ny
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


