Question: please help with part b A solution was prepared by dissolving 37.0g of KCl in 225g of water. Part A Calculate the mass percent of
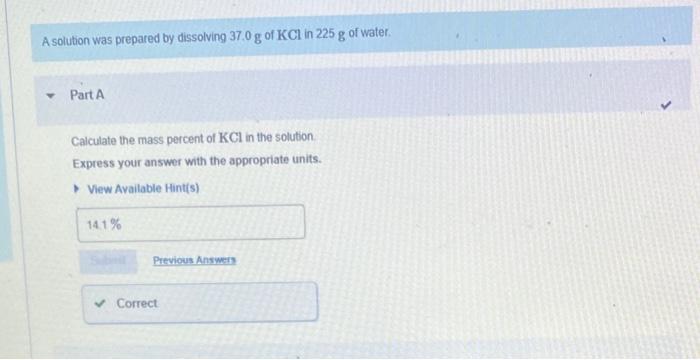
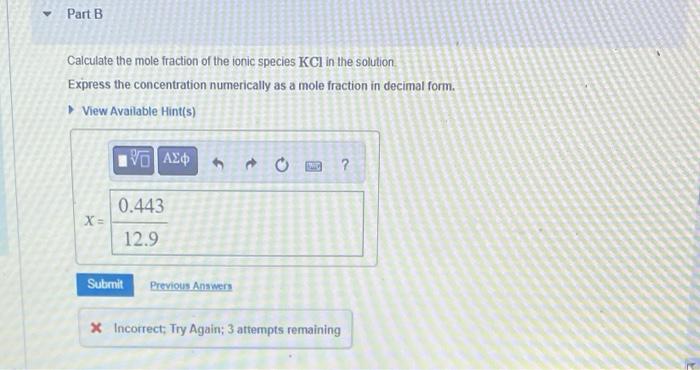
A solution was prepared by dissolving 37.0g of KCl in 225g of water. Part A Calculate the mass percent of KCl in the solution. Express your answer with the appropriate units. Calculate the mole fraction of the ionic species KCl in the solution. Express the concentration numerically as a mole fraction in decimal form
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


