Question: Please help with the QM question below: This problem is meant to justify our use of nonTeiat'ivist'ie quantum mechanics for hydrogen In class we solved
Please help with the QM question below:
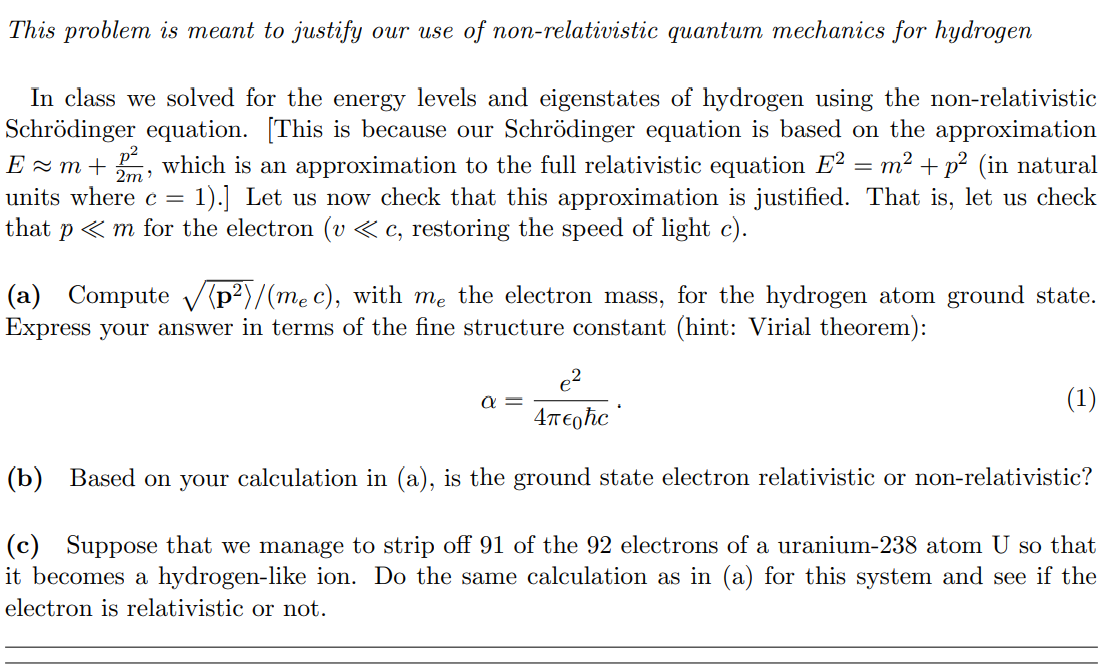
This problem is meant to justify our use of nonTeiat'ivist'ie quantum mechanics for hydrogen In class we solved for the energy levels and eigenstates of hydrogen using the nonrelativistic Schrodinger equation. [This is because our Schrodinger equation is based on the approximation E m m + %:1, which is an approximation to the full relativistic equation E2 = m2 + p2 (in natural units where c = 1).] Let us now check that this approximation is justied. That is, let us check that p
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


