Question: Please show the full Matlab code to solve this problem thank you 3. Reactor design with a single reaction (10 points) Determine the catalyst weight
Please show the full Matlab code to solve this problem
thank you
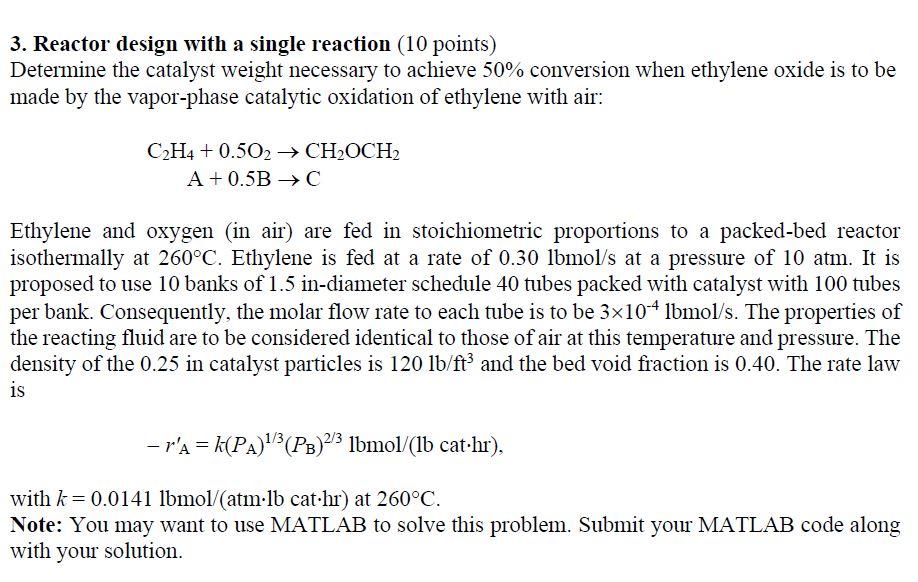
3. Reactor design with a single reaction (10 points) Determine the catalyst weight necessary to achieve 50% conversion when ethylene oxide is to be made by the vapor-phase catalytic oxidation of ethylene with air: CH4 + 0.502 CHOCH2 A +0.5B C Ethylene and oxygen (in air) are fed in stoichiometric proportions to a packed-bed reactor isothermally at 260C. Ethylene is fed at a rate of 0.30 lbmols at a pressure of 10 atm. It is proposed to use 10 banks of 1.5 in-diameter schedule 40 tubes packed with catalyst with 100 tubes per bank. Consequently, the molar flow rate to each tube is to be 3x104 lbmol/s. The properties of the reacting fluid are to be considered identical to those of air at this temperature and pressure. The density of the 0.25 in catalyst particles is 120 lb/ft? and the bed void fraction is 0.40. The rate law is - r'a = k(PA)"}(PB)23 Ibmol/(lb cat.hr), with k= 0.0141 lbmol (atmlb cat.hr) at 260C. Note: You may want to use MATLAB to solve this problem. Submit your MATLAB code along with your solution. 3. Reactor design with a single reaction (10 points) Determine the catalyst weight necessary to achieve 50% conversion when ethylene oxide is to be made by the vapor-phase catalytic oxidation of ethylene with air: CH4 + 0.502 CHOCH2 A +0.5B C Ethylene and oxygen (in air) are fed in stoichiometric proportions to a packed-bed reactor isothermally at 260C. Ethylene is fed at a rate of 0.30 lbmols at a pressure of 10 atm. It is proposed to use 10 banks of 1.5 in-diameter schedule 40 tubes packed with catalyst with 100 tubes per bank. Consequently, the molar flow rate to each tube is to be 3x104 lbmol/s. The properties of the reacting fluid are to be considered identical to those of air at this temperature and pressure. The density of the 0.25 in catalyst particles is 120 lb/ft? and the bed void fraction is 0.40. The rate law is - r'a = k(PA)"}(PB)23 Ibmol/(lb cat.hr), with k= 0.0141 lbmol (atmlb cat.hr) at 260C. Note: You may want to use MATLAB to solve this problem. Submit your MATLAB code along with your solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


