Question: For any irreversible process the net entropy change is Infinite Negative * Positive Zero A system ........ Is a region of constant mass and
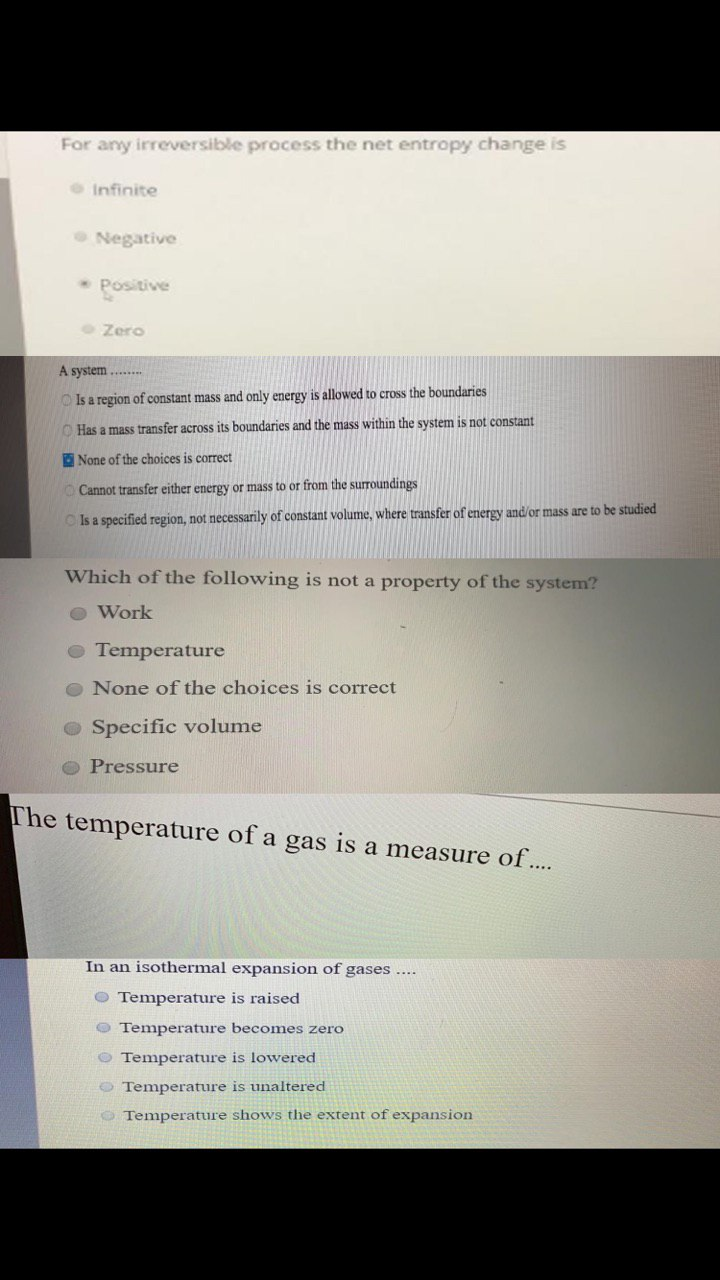
For any irreversible process the net entropy change is Infinite Negative * Positive Zero A system ........ Is a region of constant mass and only energy is allowed to cross the boundaries Has a mass transfer across its boundaries and the mass within the system is not constant None of the choices is correct Cannot transfer either energy or mass to or from the surroundings Is a specified region, not necessarily of constant volume, where transfer of energy and/or mass are to be studied Which of the following is not a property of the system? Work Temperature O None of the choices is correct Specific volume Pressure The temperature of a gas is a measure of.... In an isothermal expansion of gases .... Temperature is raised Temperature becomes zero Temperature is lowered Temperature is unaltered Temperature shows the extent of expansion
Step by Step Solution
3.55 Rating (159 Votes )
There are 3 Steps involved in it
Answer 1 Answer Positive Reason According to the second law of thermodynamics The total entropy of an isolated system that undergoes a change can neve... View full answer

Get step-by-step solutions from verified subject matter experts


