Question: please use the following format help with 1,2 and 3 How many of a solution is needed to completely react with according to the balanced
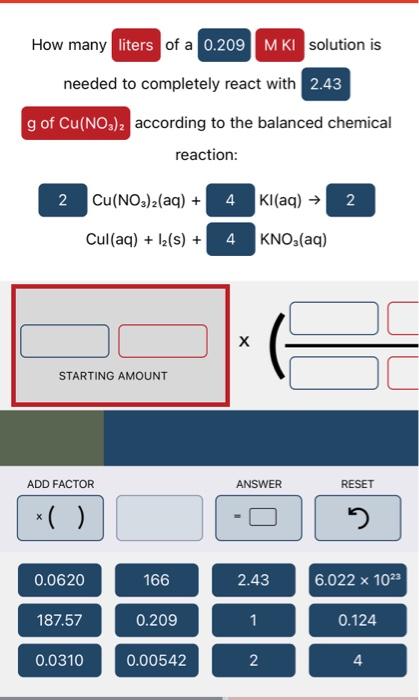
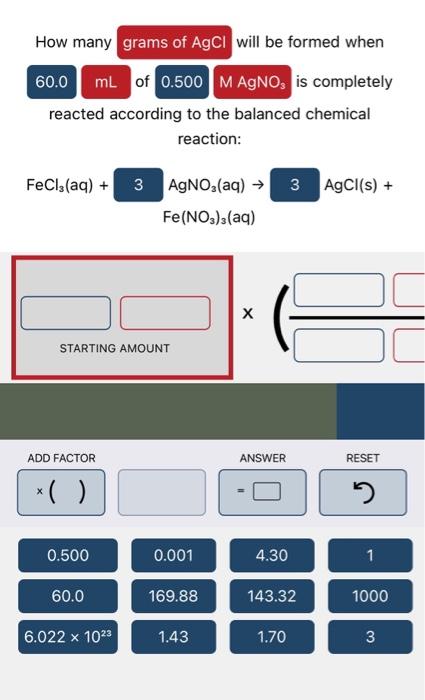
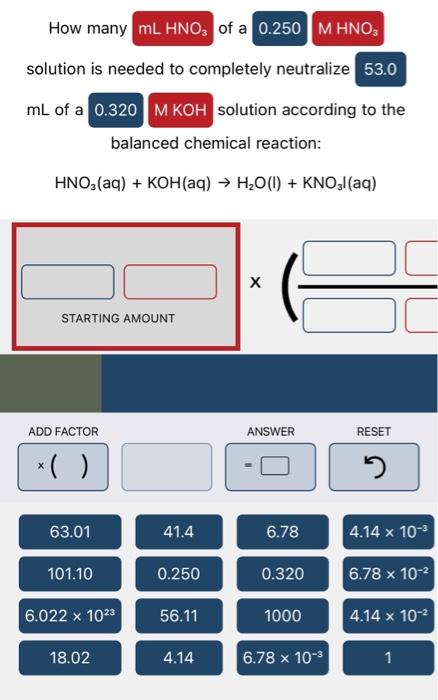
How many of a solution is needed to completely react with according to the balanced chemical reaction: Cu(NO3)2(aq)+Cul(aq)+I2(s)+Kl(aq)KNO3(aq) STARTING AMOUNT reacted according to the balanced chemical reaction: FeCl3(aq)+Fe(NO3)3(aq)AgNO3(aq) STARTING AMOUNT How many of a solution is needed to completely neutralize mL of a solution according to the balanced chemical reaction: HNO3(aq)+KOH(aq)H2O(l)+KNO3I(aq)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


