Question: Problem 1: Acentric Factor Calculation Assuming that (a) the heat of vaporization of fluid is constant, (b) the vapor phase is ideal, and (c) the
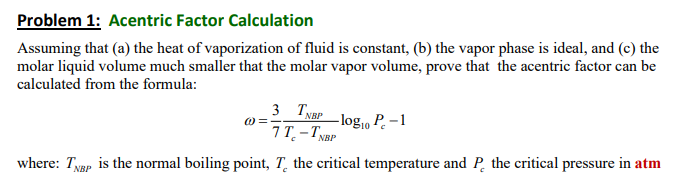
Problem 1: Acentric Factor Calculation Assuming that (a) the heat of vaporization of fluid is constant, (b) the vapor phase is ideal, and (c) the molar liquid volume much smaller that the molar vapor volume, prove that the acentric factor can be calculated from the formula: 3 TNBP O= -logo P.-1 7T-TNBP where: Txgp is the normal boiling point, T. the critical temperature and P the critical pressure in atm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


