Question: Problem 3 (25 Points) Ethylene oxide is formed in a packed bed reactor. The reaction is Along with the desired reaction the complete combustion of
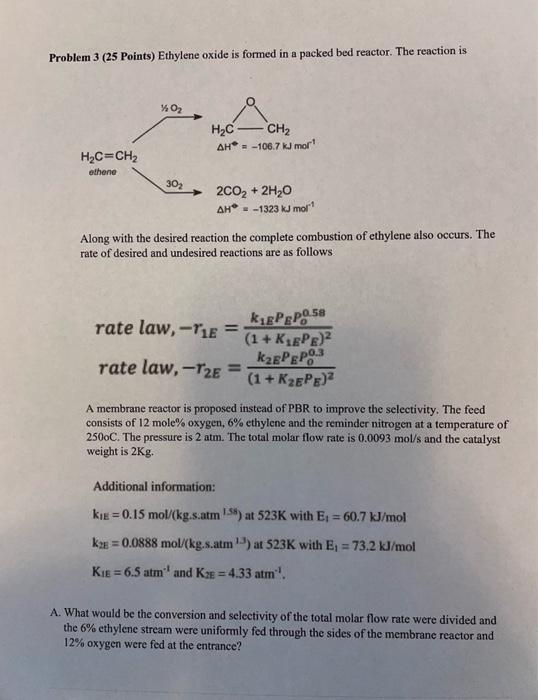
Problem 3 (25 Points) Ethylene oxide is formed in a packed bed reactor. The reaction is Along with the desired reaction the complete combustion of ethylene also occurs. The rate of desired and undesired reactions are as follows ratelaw,r2E=(1+K2EPPE)2k2EEPEP00.3 A membrane reactor is proposed instead of PBR to improve the selectivity. The feed consists of 12 mole % oxygen, 6% ethylene and the reminder nitrogen at a temperature of 2500C. The pressure is 2atm. The total molar flow rate is 0.0093mol/s and the catalyst weight is 2Kg. Additional information: K2E=0.0888mol(kgs.atm13) at 523K with E1=73.2kJ/mol K1E=6.5atm1 and K2E=4.33atm1. A. What would be the conversion and selectivity of the total molar flow rate were divided and the 6% ethylene stream were uniformly fed through the sides of the membrane reactor and 12% oxygen were fed at the entrance
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


