Question: Problem 5. A standard solution is prepared by completely dissolving 0.4 grams of CaCl2 and 0.2 grams of MgCO3 salts in 1-Liter DI water in
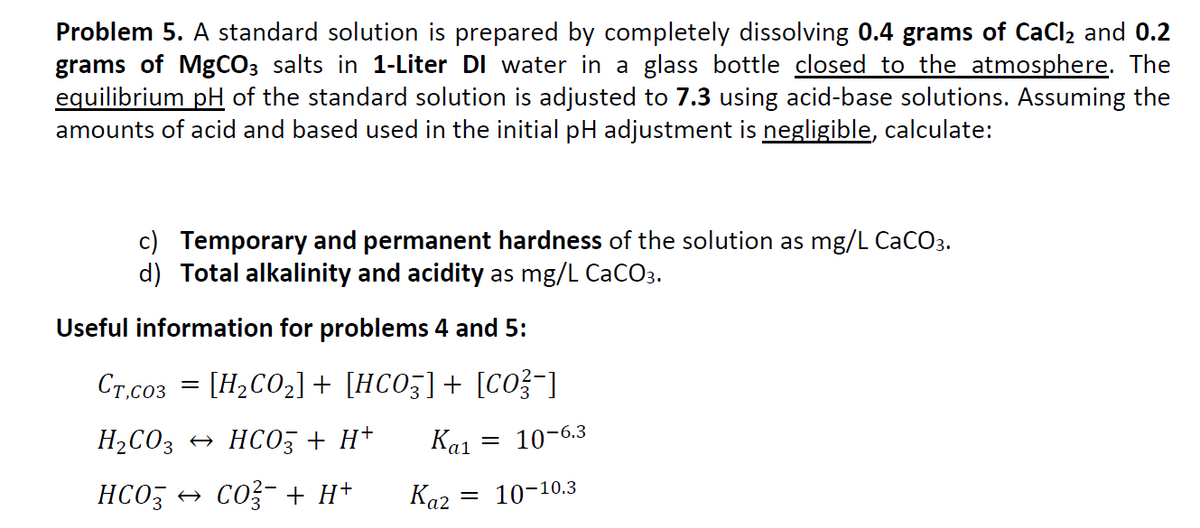
Problem 5. A standard solution is prepared by completely dissolving 0.4 grams of CaCl2 and 0.2 grams of MgCO3 salts in 1-Liter DI water in a glass bottle closed to the atmosphere. The equilibrium pH of the standard solution is adjusted to 7.3 using acid-base solutions. Assuming the amounts of acid and based used in the initial pH adjustment is negligible, calculate: c) Temporary and permanent hardness of the solution as mg/LCaCO3. d) Total alkalinity and acidity as mg/LCaCO3. Useful information for problems 4 and 5: CT,CO3=[H2CO2]+[HCO3]+[CO32]H2CO3HCO3+H+Ka1=106.3HCO3CO32+H+Ka2=1010.3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


