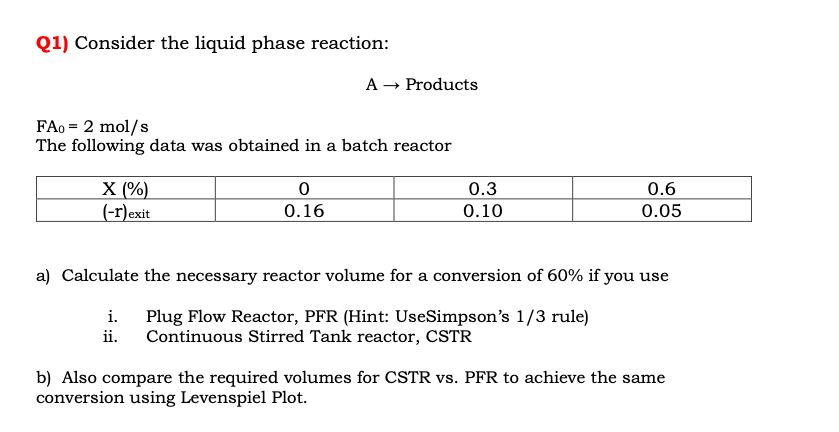
Question: Q 1 ) Consider the liquid phase reaction: A Products F A 0 = 2 m o l s The following data was obtained in
Q Consider the liquid phase reaction:
Products
The following data was obtained in a batch reactor
a Calculate the necessary reactor volume for a conversion of if you use
i Plug Flow Reactor, PFR Hint: UseSimpson's rule
ii Continuous Stirred Tank reactor, CSTR
b Also compare the required volumes for CSTR vs PFR to achieve the same
conversion using Levenspiel Plot.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


