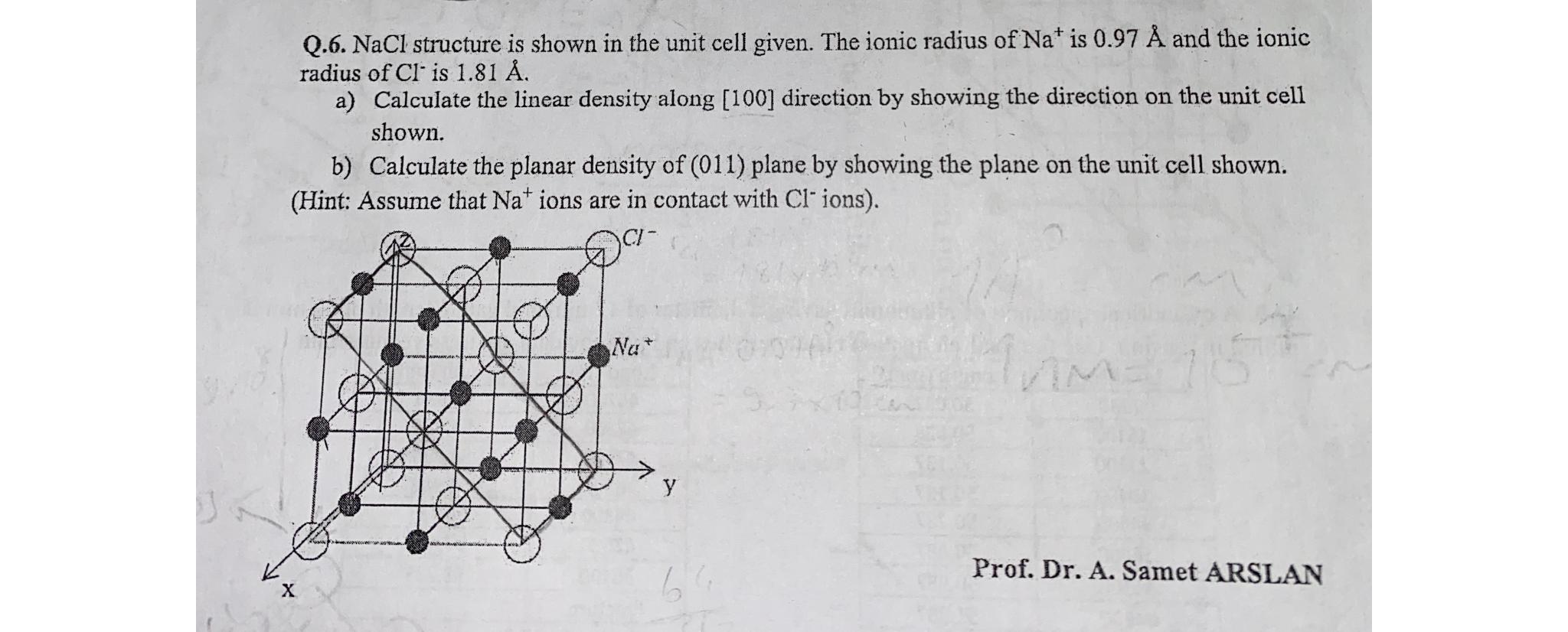
Question: Q . 6 . NaCl structure is shown in the unit cell given. The ionic radius of N a + is 0 . 9 7
Q NaCl structure is shown in the unit cell given. The ionic radius of is and the ionic radius of is
a Calculate the linear density along direction by showing the direction on the unit cell shown.
b Calculate the planar density of plane by showing the plane on the unit cell shown.
Hint: Assume that ions are in contact with ions
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


