Question: X QNaCl structure is shown in the unit cell given. The ionic radius of Nat is 0.97 and the ionic radius of Cl is
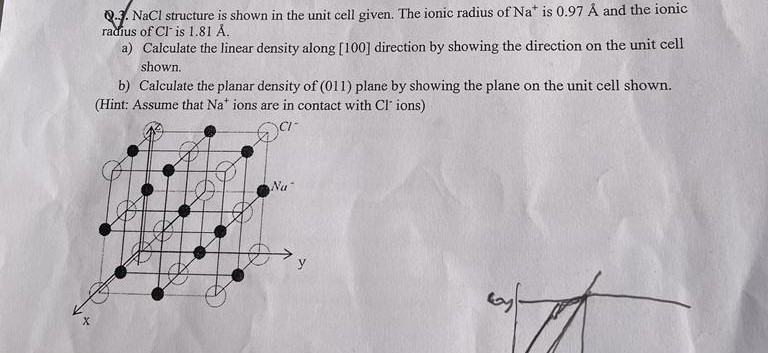
X QNaCl structure is shown in the unit cell given. The ionic radius of Nat is 0.97 and the ionic radius of Cl is 1.81 . a) Calculate the linear density along [100] direction by showing the direction on the unit cell shown. b) Calculate the planar density of (011) plane by showing the plane on the unit cell shown. (Hint: Assume that Na* ions are in contact with Cl' ions) Na y A
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


