Question: Question 2: For a system 10 M Cr3+ in hydrochloric acid at a pH of 1: (a) Determine the corrosion current and potential. (b) Evaluate
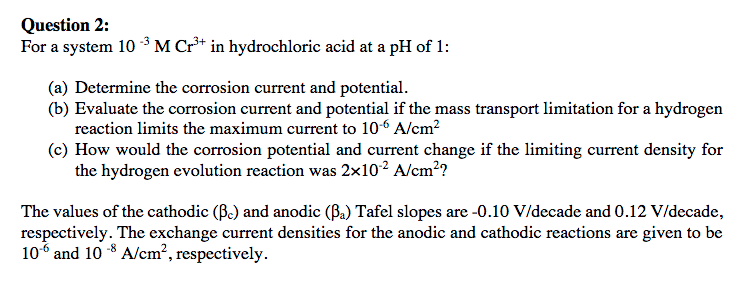
Question 2: For a system 10 M Cr3+ in hydrochloric acid at a pH of 1: (a) Determine the corrosion current and potential. (b) Evaluate the corrosion current and potential if the mass transport limitation for a hydrogen reaction limits the maximum current to 10-6 A/cm2 (c) How would the corrosion potential and current change if the limiting current density for the hydrogen evolution reaction was 2x102 A/cm?? The values of the cathodic (B.) and anodic (Ba) Tafel slopes are -0.10 V/decade and 0.12 V/decade, respectively. The exchange current densities for the anodic and cathodic reactions are given to be 10 and 10 -8 A/cm, respectively
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


