Question: Apply the Gibbs phase rule to determine the number of independent primary reactions needed to calculate the equilibrium composition of the butadiene system at
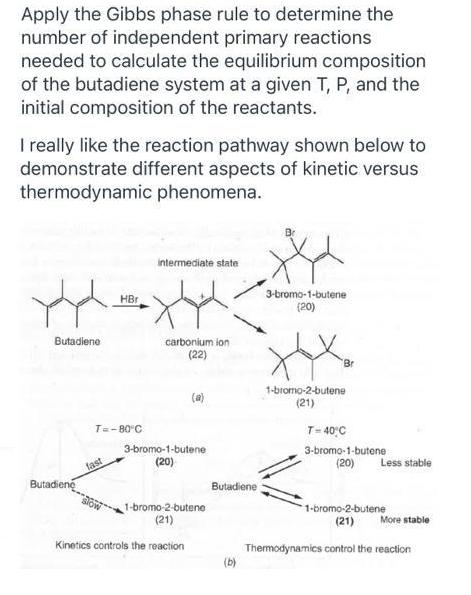
Apply the Gibbs phase rule to determine the number of independent primary reactions needed to calculate the equilibrium composition of the butadiene system at a given T, P, and the initial composition of the reactants. I really like the reaction pathway shown below to demonstrate different aspects of kinetic versus thermodynamic phenomena. intermediate state 3-bromo-1-butene (20) HBr Butadiene carbonium ion (22) Br 1-bromo-2-butene (21) (a) T=-80 C T- 40C 3-bromo-1-butene 3-bromo-1-butene (20) fast (20) Less stable Butadiene Butadiene 1-bromo-2-butene (21) 1-bromo-2-butene (21) More stable Kinetics controls the reaction Thermodynamics control the reaction (b)
Step by Step Solution
3.48 Rating (161 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


