Question: Question 6 (Adiabatic and polytropic operation) 15 points You study an endothermic reaction in a CSTR. (4P) 1) Assume that the adiabatic temperature change is
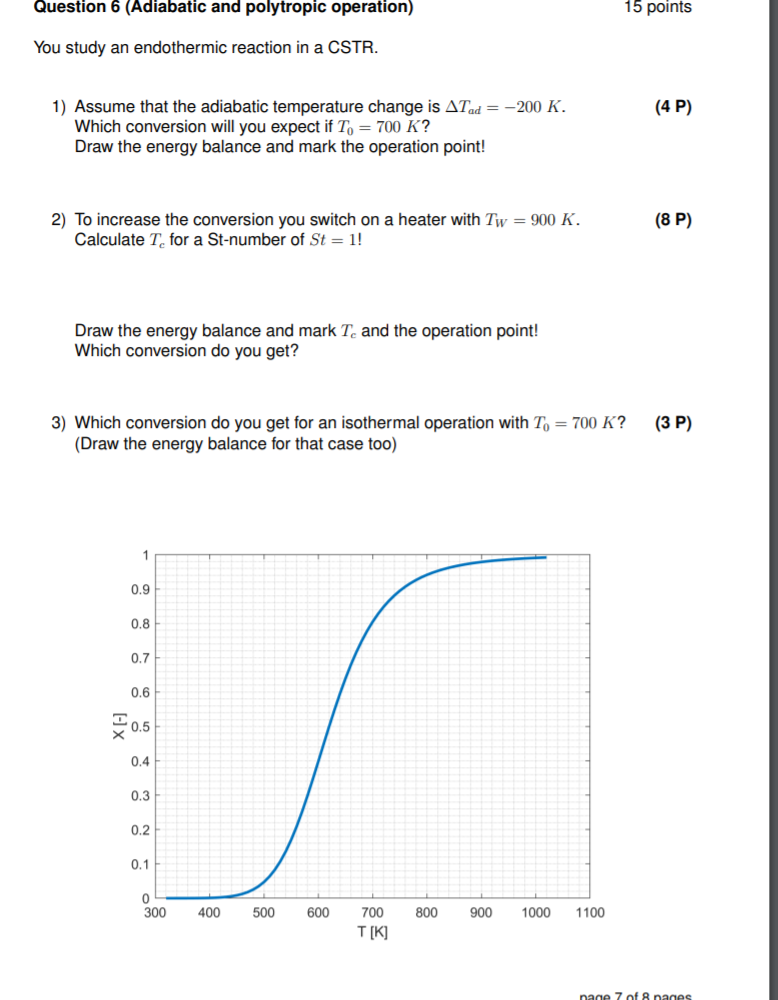
Question 6 (Adiabatic and polytropic operation) 15 points You study an endothermic reaction in a CSTR. (4P) 1) Assume that the adiabatic temperature change is Arad = -200 K. Which conversion will you expect if To = 700 K? Draw the energy balance and mark the operation point! (8 P) 2) To increase the conversion you switch on a heater with Tw = 900 K. Calculate T for a St-number of St = 1! Draw the energy balance and mark T. and the operation point! Which conversion do you get? (3P) 3) Which conversion do you get for an isothermal operation with To = 700 K? (Draw the energy balance for that case too) 1 0.9 0.8 0.7 0.6 20.5 0.4 0.3 0.2 0.1 0 300 400 500 600 800 900 1000 1100 700 T[K] nage 7 of 8 nanes
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


