Question: Questions: 1. Which ions were present in your unknown solution? Explain how you know. 2. Write the net ionic equation for each of the possible

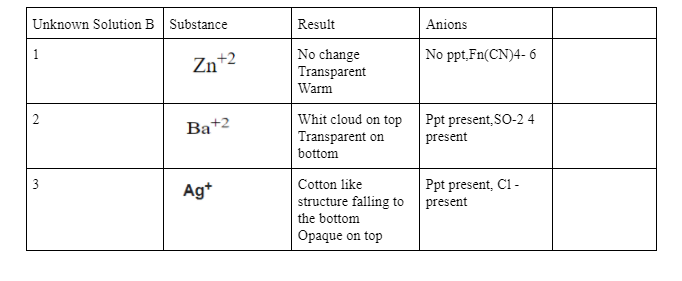
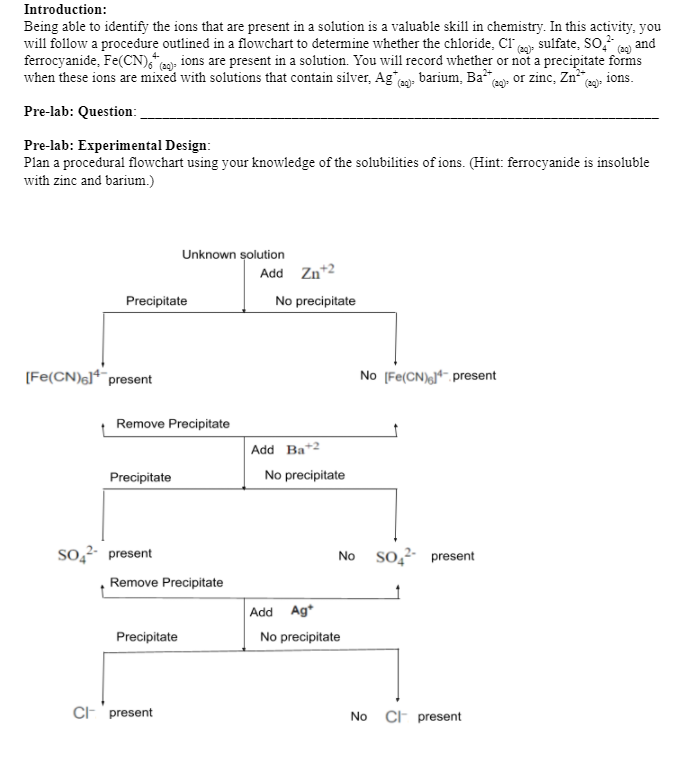
Questions: 1. Which ions were present in your unknown solution? Explain how you know. 2. Write the net ionic equation for each of the possible reactions in this experiment. Assume the anions where accompanied by sodium. 3. When selecting the first chemical to add to the solution in order to identify the ions present, what must this chemical do and what must it not do? 4. If the chemical you are adding is a cation, which anion must accompany it? Why? 5. Suggest sources of error and describe changes to your procedure that would help to reduce these sources of error. 6. Explain how what you learned in this lab can be applied to water treatment and quality testing. Give specific examples. Unknown Solution B Substance Anions No ppt,Fn(CN)4-6 Zn+2 Result No change Transparent Warm Whit cloud on top Transparent on bottom 2 Ba+2 Ppt present SO-24 present 3 Agt Cotton like structure falling to the bottom Opaque on top Ppt present, CI - present (20) Introduction: Being able to identify the ions that are present in a solution is a valuable skill in chemistry. In this activity, you will follow a procedure outlined in a flowchart to determine whether the chloride, Cr(2), sulfate, So: (a) and ferrocyanide, Fe(CN). a), ions are present in a solution. You will record whether or not a precipitate forms when these ions are mixed with solutions that contain silver, Aga). barium. Bacaq). Or zinc, Zn ions. Pre-lab: Question: Pre-lab: Experimental Design: Plan a procedural flowchart using your knowledge of the solubilities of ions. (Hint: ferrocyanide is insoluble with zinc and barium.) Unknown solution Add Zn+2 Precipitate No precipitate [Fe(CN)614 present No [Fe(CN),14 present Remove Precipitate Add Ba+2 Precipitate No precipitate SO42- present Remove Precipitate No SO42- present Add Ag No precipitate Precipitate Cl present No C present
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


