Question: read experiment attached Q: Using the Table of Standard Reduction Potentials, calculate the standard cell voltage, E, for each of the cells tested. Compare the
read experiment attached
Q: Using the Table of Standard Reduction Potentials, calculate the standard cell voltage, E, for each of the cells tested. Compare the observed and calculated values. How can you account for the differences? Write the shorthand notation for each cell and write the anode and cathode reactions and also the net ionic equation of the overall redox reaction. Explain the function of the salt bridge.
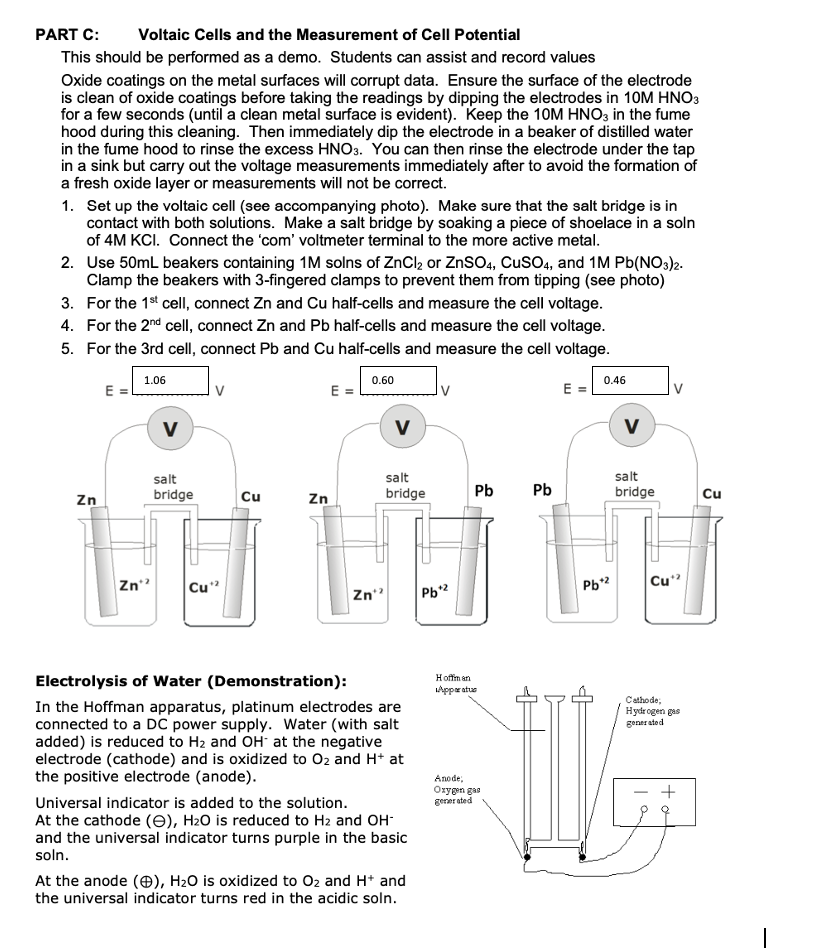
PART C: Voltaic Cells and the Measurement of Cell Potential This should be performed as a demo. Students can assist and record values Oxide coatings on the metal surfaces will corrupt data. Ensure the surface of the electrode is clean of oxide coatings before taking the readings by dipping the electrodes in 10MHNO3 for a few seconds (until a clean metal surface is evident). Keep the 10MHNO3 in the fume hood during this cleaning. Then immediately dip the electrode in a beaker of distilled water in the fume hood to rinse the excess HNO3. You can then rinse the electrode under the tap in a sink but carry out the voltage measurements immediately after to avoid the formation of a fresh oxide layer or measurements will not be correct. 1. Set up the voltaic cell (see accompanying photo). Make sure that the salt bridge is in contact with both solutions. Make a salt bridge by soaking a piece of shoelace in a soln of 4MKCl. Connect the 'com' voltmeter terminal to the more active metal. 2. Use 50mL beakers containing 1M solns of ZnCl2 or ZnSO4,CuSO4, and 1MPb(NO3)2. Clamp the beakers with 3 -fingered clamps to prevent them from tipping (see photo) 3. For the 1st cell, connect Zn and Cu half-cells and measure the cell voltage. 4. For the 2nd cell, connect Zn and Pb half-cells and measure the cell voltage. 5. For the 3rd cell, connect Pb and Cu half-cells and measure the cell voltage. E=VVE=VE=V=V Electrolysis of Water (Demonstration): In the Hoffman apparatus, platinum electrodes are connected to a DC power supply. Water (with salt added) is reduced to H2 and OHat the negative electrode (cathode) and is oxidized to O2 and H+at the positive electrode (anode). Universal indicator is added to the solution. At the cathode (),H2O is reduced to H2 and OH and the universal indicator turns purple in the basic soln. At the anode (),H2O is oxidized to O2 and H+and the universal indicator turns red in the acidic soln
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


