Question: Selective Oxidation The standard reduction potential for the half-reaction Sn4+ + 2e - Sn2+ is +0.15 V. Consider data from the table of standard reduction
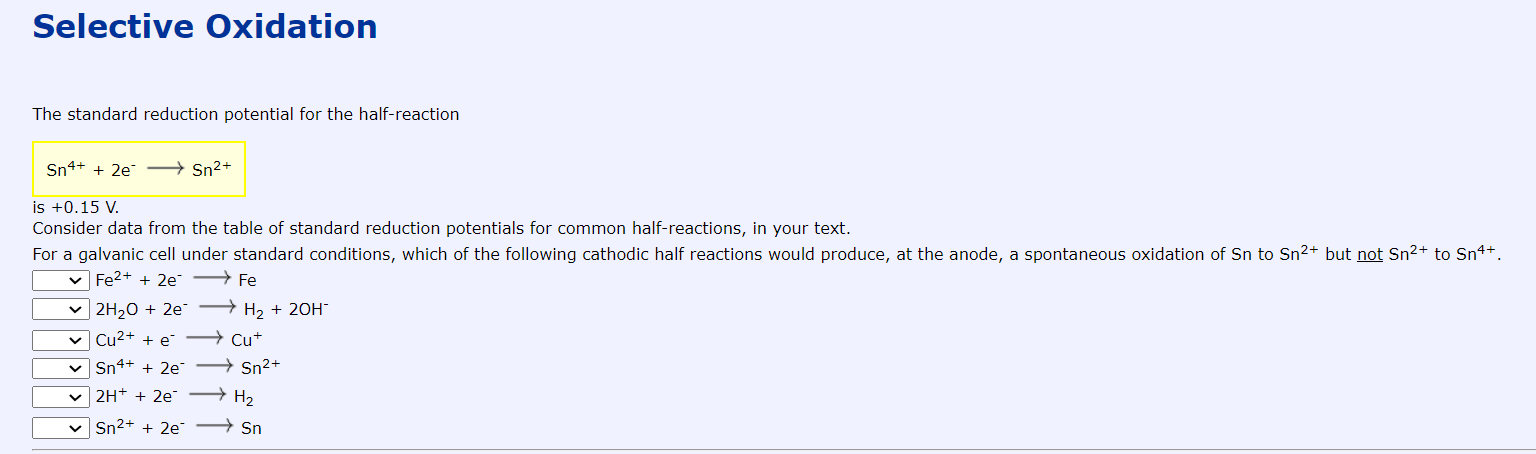
Selective Oxidation The standard reduction potential for the half-reaction Sn4+ + 2e - Sn2+ is +0.15 V. Consider data from the table of standard reduction potentials for common half-reactions, in your text. For a galvanic cell under standard conditions, which of the following cathodic half reactions would produce, at the anode, a spontaneous oxidation of Sn to Sn2+ but not Sn2+ to Sn4+. Fe2+ + 2e - Fe 2H20 + 2 H2 + 20H Cu2+ + e - Cut Sn4+ + 2e Sn2+ 2H+ + 2e - H2 Sn2+ + 2e-Sn Selective Oxidation The standard reduction potential for the half-reaction Sn4+ + 2e - Sn2+ is +0.15 V. Consider data from the table of standard reduction potentials for common half-reactions, in your text. For a galvanic cell under standard conditions, which of the following cathodic half reactions would produce, at the anode, a spontaneous oxidation of Sn to Sn2+ but not Sn2+ to Sn4+. Fe2+ + 2e - Fe 2H20 + 2 H2 + 20H Cu2+ + e - Cut Sn4+ + 2e Sn2+ 2H+ + 2e - H2 Sn2+ + 2e-Sn
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


