Question: Use data from Table 19.1 to predict whether, to any significant extent, (a) Mg(s) will displace Pb 2+ from aqueous solution; (b) Sn(s) will react
Use data from Table 19.1 to predict whether, to any significant extent,
(a) Mg(s) will displace Pb2+ from aqueous solution;
(b) Sn(s) will react with and dissolve in 1 M HCl;
(c) SO42- will oxidize Sn2+ to Sn4+ in acidic solution;
(d) MnO4-(aq) will oxidize H2O2(aq) to O2(g) in acidic solution;
(e) I2(s) will displace Br-(aq) to produce Br2(l).
Table 19.1
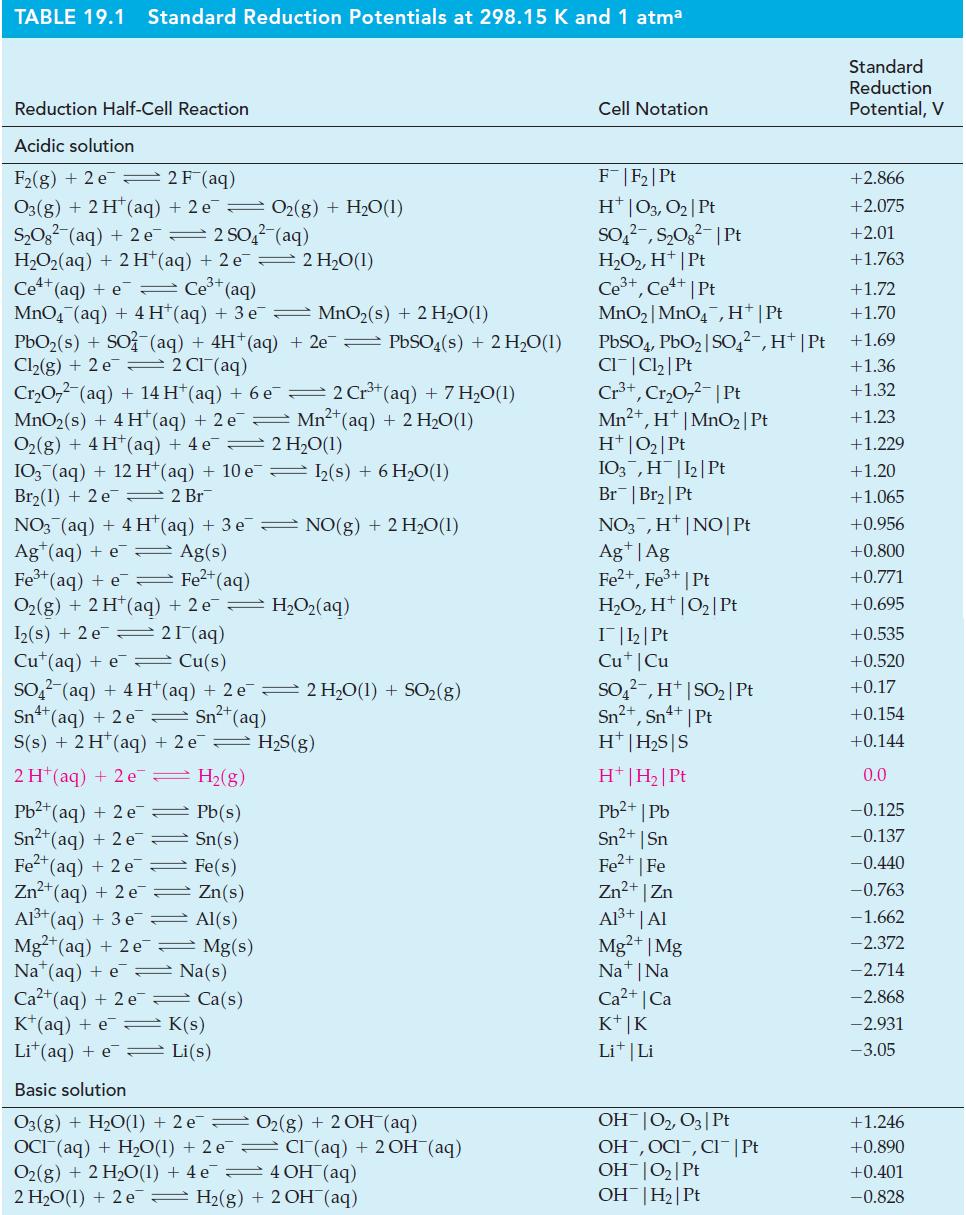
TABLE 19.1 Standard Reduction Potentials at 298.15 K and 1 atma Reduction Half-Cell Reaction Acidic solution F(g) + 2 e 2 F (aq) O3(g) + 2 H+ (aq) + 2 e O(g) + HO(1) SO (aq) + 2 e 2 SO4(aq) H,Oz(aq) + 2H*(aq) +2e
Step by Step Solution
3.39 Rating (168 Votes )
There are 3 Steps involved in it
To predict whether certain reactions will occur you can compare the standard reduction potentials of ... View full answer

Get step-by-step solutions from verified subject matter experts


