Question: Solid copper(I) chloride has a crystal structure with the cubic unit cell pictured below, where the Clions are represented as green spheres. How many Clions
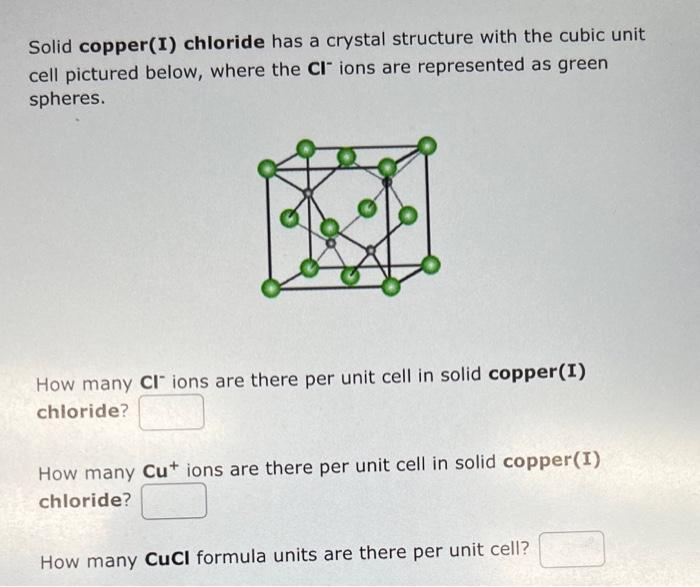
Solid copper(I) chloride has a crystal structure with the cubic unit cell pictured below, where the Clions are represented as green spheres. How many Clions are there per unit cell in solid copper(I) chloride? How many Cu+ions are there per unit cell in solid copper(I) chloride? How many CuCl formula units are there per unit cell
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


