Question: standard reduction potential table. 5 . Write a balanced molecular equation for the Red - Ox reaction, hydrolysis of the sait and metal reaction with
standard reduction potential table.
Write a balanced molecular equation for the RedOx reaction, hydrolysis of the sait and metal reaction with hydrolysis product:
A galvanic cell is set up as follows:
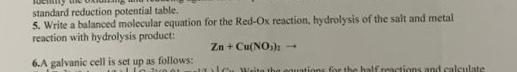
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


