Question: Starting from the triple point (T = -56.7 C and P = 5.1 atm), calculate the vapour pressure of liquid CO 2 at 31.0 C.
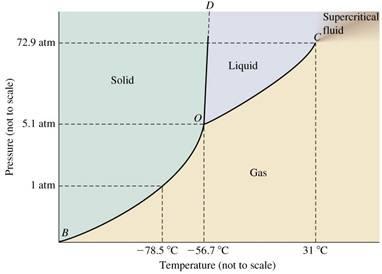
Starting from the triple point (T = -56.7 C and P = 5.1 atm), calculate the vapour pressure of liquid CO2 at 31.0 C. vapH = 15.326 kJ mol-1 for CO2
D Supercritical fluid 72.9 atm Liquid Solid Pressure (not to scale) 5.1 atm Gas 1 atm B 31 C -78.5C -56.7 C Temperature (not to scale)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


