Question: STEP BY STEP SOLUTION,PLEASE!! A rotameter was calibrated with N 2 at T (ref) =293 K and P ref = 760 mmHg. Molecular weight of
STEP BY STEP SOLUTION,PLEASE!!
A rotameter was calibrated with N2 at T(ref)=293 K and Pref= 760 mmHg. Molecular weight of nitrogen N2 (gas)=28 g/mol.
Volumetric flow rate of nitrogen passing through the rotameter QN2=500 ml/min. In a later study, hydrogen gas was passed through the same rotameter. Find the volumetric flow rate QH2 of hydrogen for the following conditions.
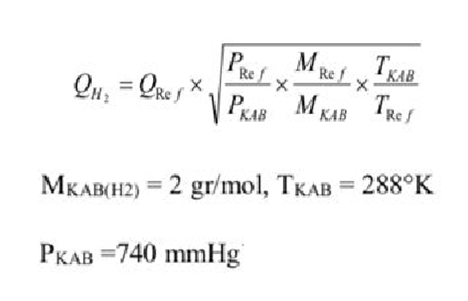
QH2=QRefPKABPRefMKABMRefTRefTKABMKAB(H2)=2gr/mol,TKAB=288KPKAB=740mmHg
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


