Question: The data below were collected for the folowing reaction: Write an expression for the reaction rate law. Rate=k[NO2]Rate=k[F2]Rate=k[NO2][F2]Rate=k[NO2]2[F2] Part B Calculate the value of the
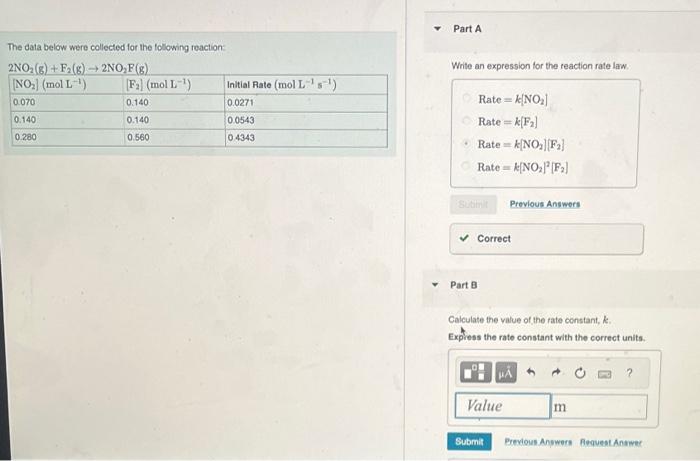
The data below were collected for the folowing reaction: Write an expression for the reaction rate law. Rate=k[NO2]Rate=k[F2]Rate=k[NO2][F2]Rate=k[NO2]2[F2] Part B Calculate the value of the rate constant, k. Expless the rate constant with the correct units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


