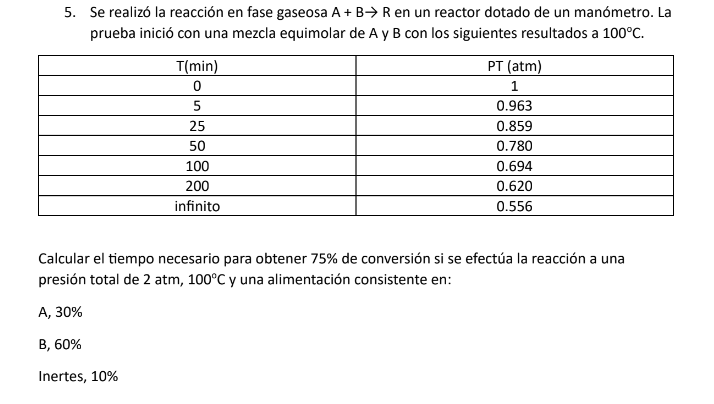
Question: The gas phase reaction A + B - > R was performed in a reactor equipped with a pressure gauge. The test started with an
The gas phase reaction A B R was performed in a reactor equipped with a pressure gauge. The test started with an equimolar mixture of A and B with the following results at deg C
Calculate the time required to achieve a conversion if the reaction is carried out at a total pressure of atm, deg C and a feed consisting of:
A
B
Inert,
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


