Question: The graph above represents the titration of a weak base with HCl. Fill in the blanks with the letter corresponding to the appropriate point on
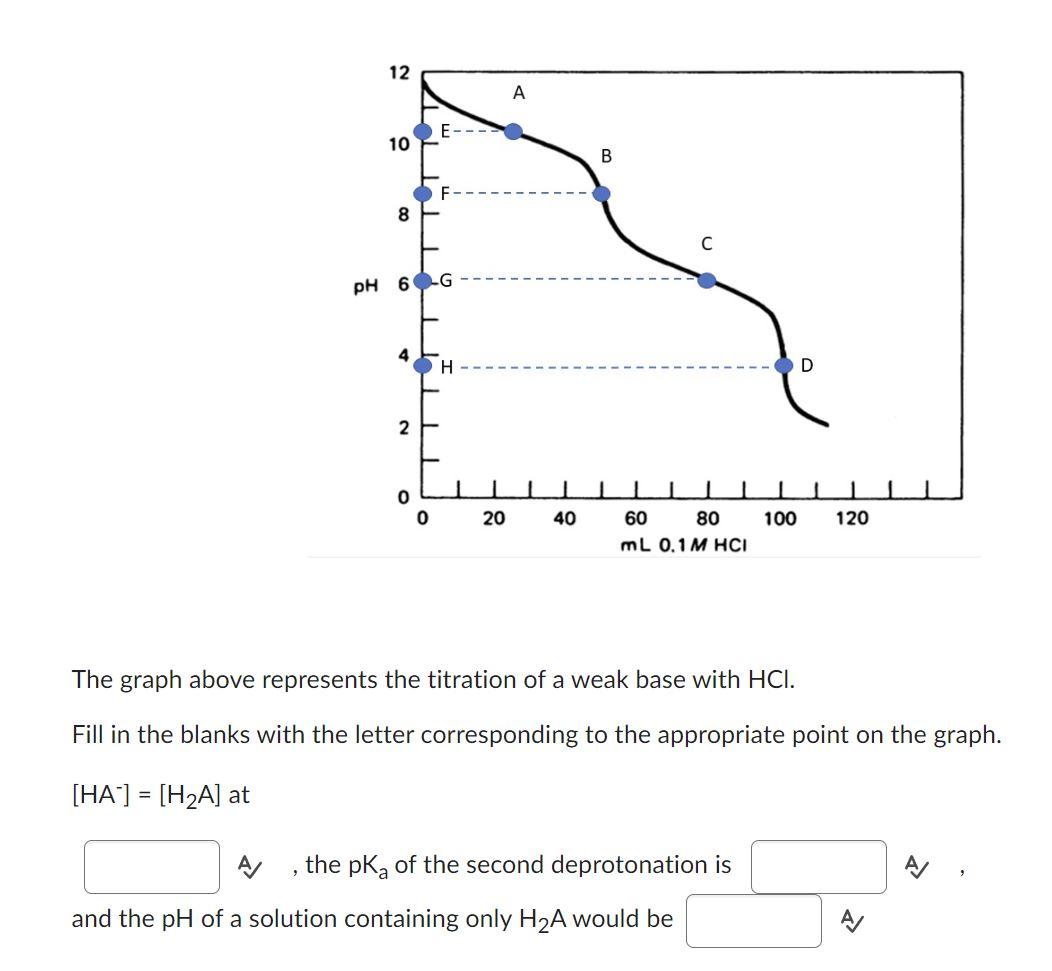
The graph above represents the titration of a weak base with HCl. Fill in the blanks with the letter corresponding to the appropriate point on the graph. [HA]=[H2A] at A , the pKa of the second deprotonation is A and the pH of a solution containing only H2A would be 4
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


