Question: The ionization percentage of weak acid decrease as acid concentration increase : One of the following reactions has K value larger than 1 : H2S+SO42HS+HSO4NH4++H2PO4NH3+H3PO4HF+NH3NH4++FH2CO3+FHF+HCO3
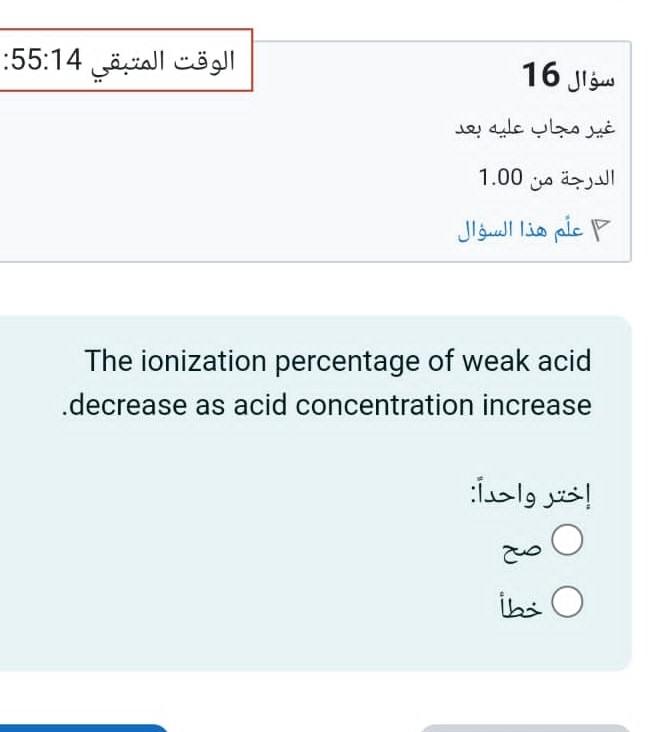
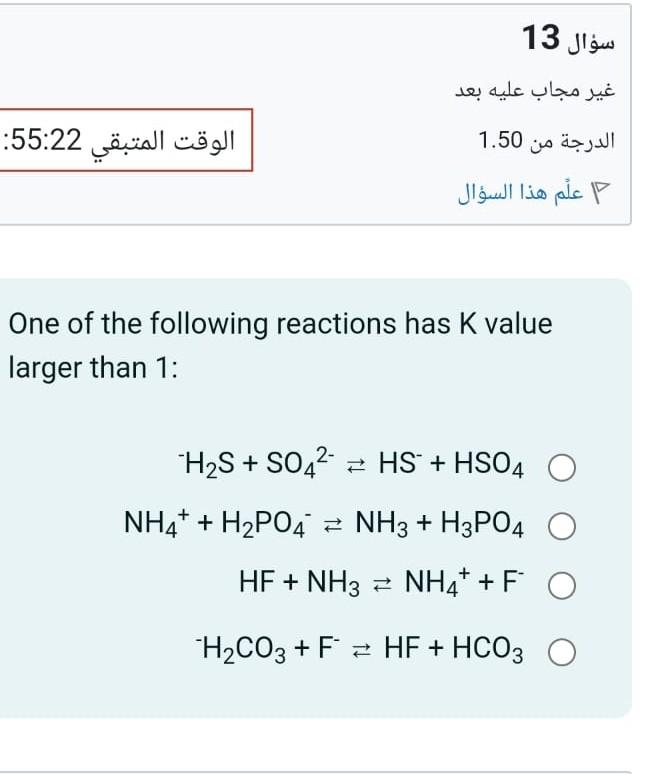
The ionization percentage of weak acid decrease as acid concentration increase : One of the following reactions has K value larger than 1 : H2S+SO42HS+HSO4NH4++H2PO4NH3+H3PO4HF+NH3NH4++FH2CO3+FHF+HCO3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


