Question: use Ecell = Enernst + Ej calculate Ej with Henderson eqn. answer this correctly 2.4 What are the cell reactions and their emfs in the
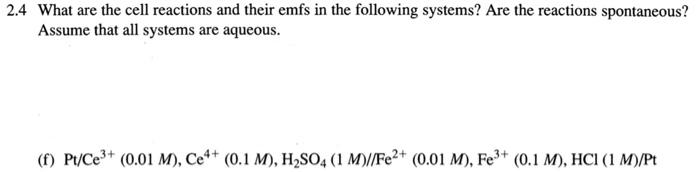
2.4 What are the cell reactions and their emfs in the following systems? Are the reactions spontaneous? Assume that all systems are aqueous. (f) Pt/Ce3+ (0.01 M), Ce4+ (0.1 M), H2SO4 (1 M)//Fe2+ (0.01 M), Fe3+ (0.1 M), HCI (1 M)/Pt
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


