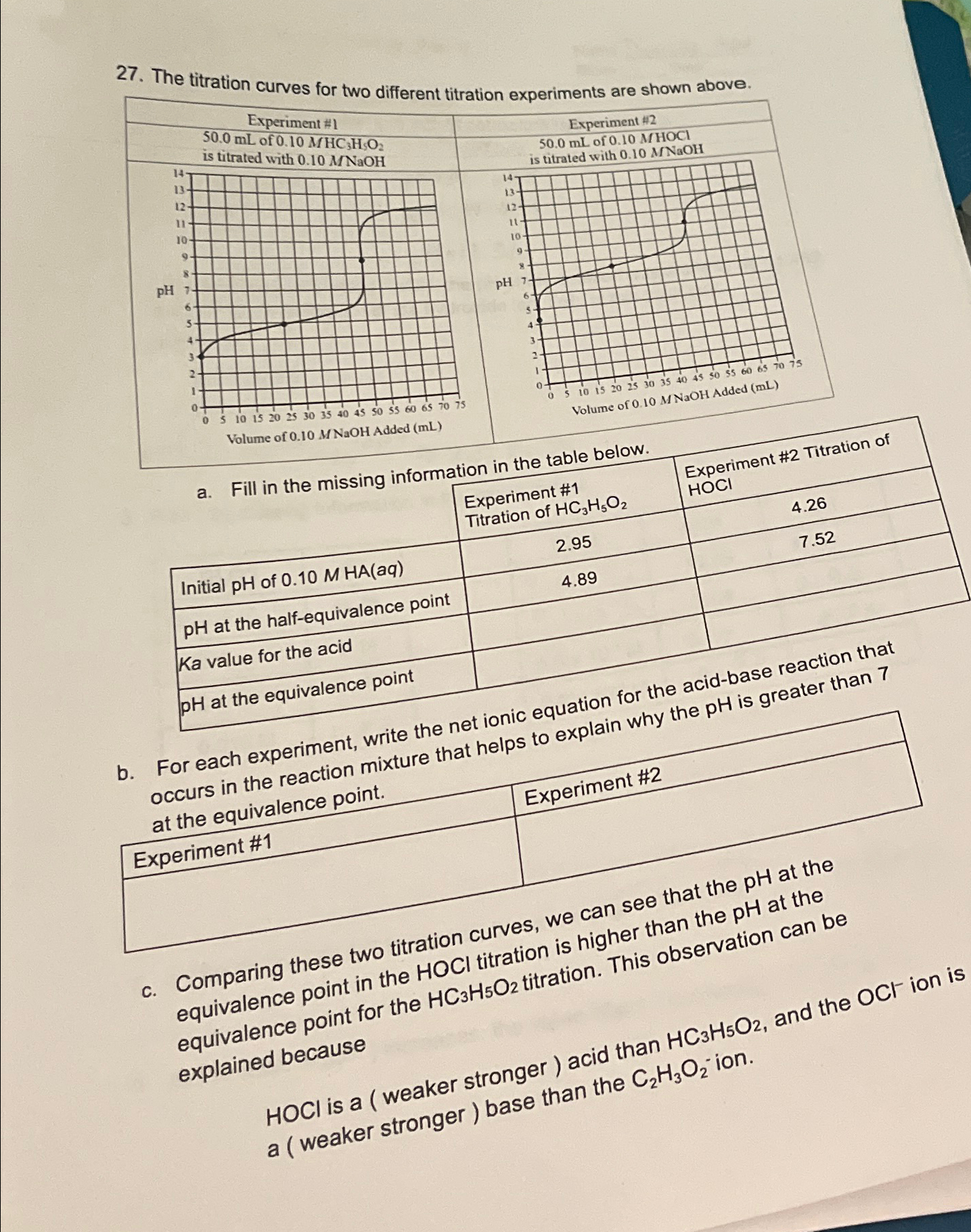
Question: The titration curves for two differant titration aynariments are shown above. a . Fill in the missing information in the table wo . - of
The titration curves for two differant titration aynariments are shown above.
a Fill in the missing information in the table wo
of
at the equivalence point
b
h experiment, write the net ionic equation for the acidbase reaction that re that helps to explain why the is greater than
c Comparing these two titration curves, we car. equivalence point in the HOCl titration is higher than the equivalence point for the titration. This observation can be explained because
HOCl is a weaker stronger acid than and the OCl ion is a weaker stronger base than the ion.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


