Question: This exercise will lead you to verify that the average atomic mass of magnesium is 24.31 amu, based on the following information: The average atomic
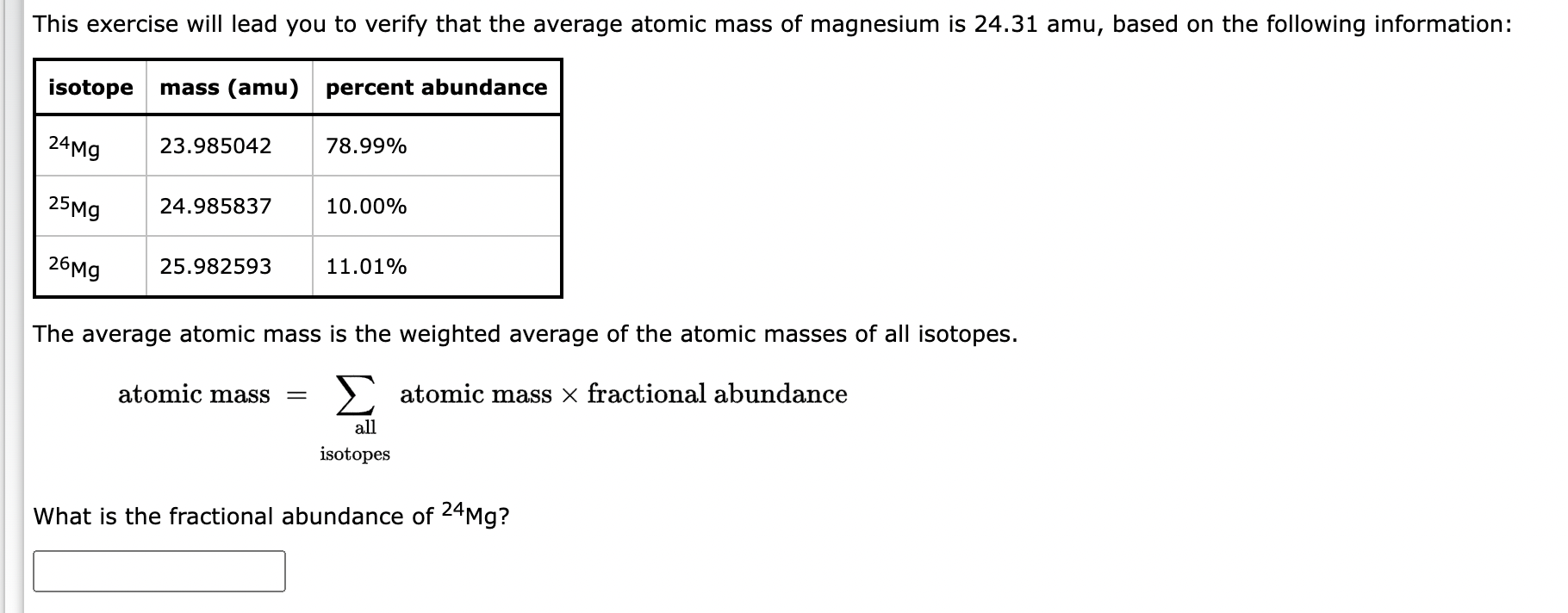
This exercise will lead you to verify that the average atomic mass of magnesium is 24.31 amu, based on the following information: The average atomic mass is the weighted average of the atomic masses of all isotopes. atomicmass=allatomicmassfractionalabundance isotopes What is the fractional abundance of 24Mg
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


