Question: THIS IS A THREE PART QUESTION. THIS IS PART 3 Hydrogen gas can be produced by reacting iron with a solution of hydrochloric acid. The
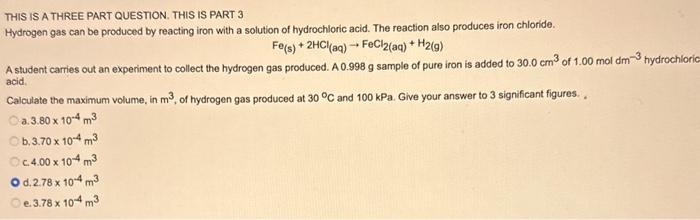
THIS IS A THREE PART QUESTION. THIS IS PART 3 Hydrogen gas can be produced by reacting iron with a solution of hydrochloric acid. The reaction also produces iron chloride. Fe(s)+2HCl(aq)FeCl2(aq)+H2(g) A student carries out an experiment to collect the hydrogen gas produced. A 0.998g sample of pure iron is added to 30.0cm3 of 1.00moldm3hydrochlor2 acid. Calculate the maximum volume, in m3, of hydrogen gas produced at 30C and 100kPa. Give your answer to 3 significant figures. . a. 3.80104m3 b. 3.70104m3 c. 4.00104m3 d. 2.78104m3 e. 3.78104m3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


