Question: This molecule (A) can behave as both an acid and a base. It is acidic because it is capable of losing a proton (H+)to form
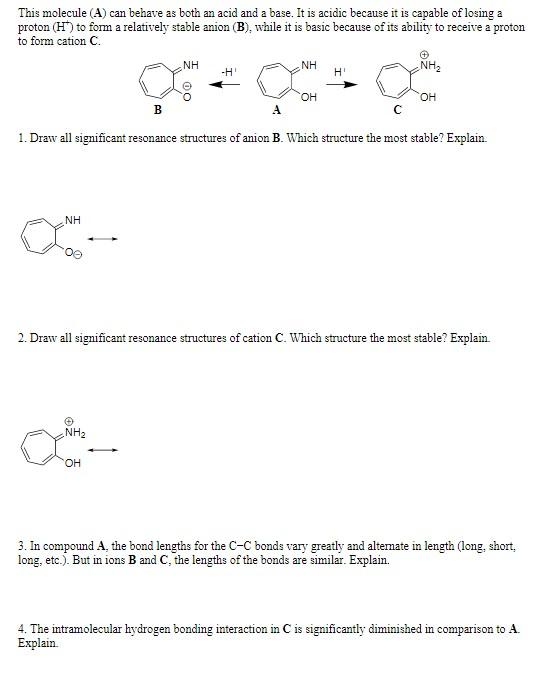
This molecule (A) can behave as both an acid and a base. It is acidic because it is capable of losing a proton (H+)to form a relatively stable anion (B), while it is basic because of its ability to receive a proton to form cation C. HI 1. Draw all significant resonance structures of anion B. Which structure the most stable? Explain. 2. Draw all significant resonance structures of cation C. Which structure the most stable? Explain. 3. In compound A, the bond lengths for the CC bonds vary greatly and alternate in length (long, short, long, etc.). But in ions B and C, the lengths of the bonds are similar. Explain. 4. The intramolecular hydrogen bonding interaction in C is significantly diminished in comparison to A. Explain
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


