Question: TRvw Tople Use the References to access important values if needed for the queste The following thermochemical equation is for the reaction of hydrogen bromide(g)
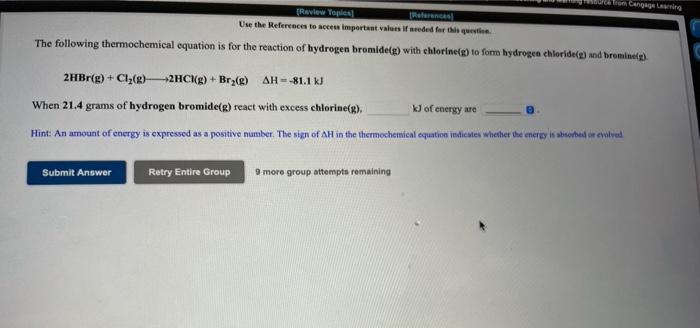
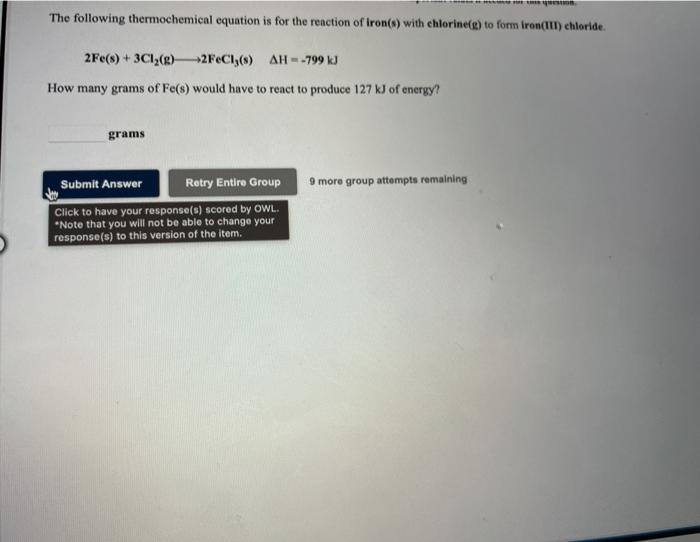
TRvw Tople Use the References to access important values if needed for the queste The following thermochemical equation is for the reaction of hydrogen bromide(g) with chlorine(g) to form hydrogen chloridele) and brominelp). 2HBr(g) + Cl2(e) -2HC (g) + Bry() AH = -81.1 KJ When 21.4 grams of hydrogen bromide(g) react with excess chlorine(e), J of energy are Hint: An amount of energy is expressed as a positive number. The sign of AH in the thermochemical equation indicates whether the energy is absorbed or evolved Submit Answer Retry Entire Group 9 moro group attempts remaining The following thermochemical equation is for the reaction of iron(s) with chlorinele) to form Iron(III) chloride. 2Fe(s) + 3Cl2(8) 2FeC138) AH--799 kJ How many grams of Fe(s) would have to react to produce 127 kJ of energy? grams 9 more group attempts remaining Submit Answer Retry Entire Group Click to have your response(s) scored by OWL. *Note that you will not be able to change your response(s) to this version of the item
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


