Question: Use the References to access important values if needed for this question. A sample of 3.240 grams of a compound containing carbon and hydrogen reacts
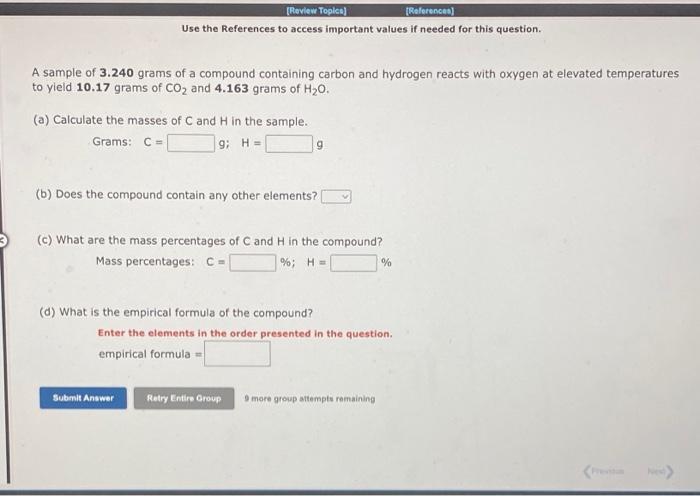
Use the References to access important values if needed for this question. A sample of 3.240 grams of a compound containing carbon and hydrogen reacts with oxygen at elevated temperatures to yield 10.17grams of CO2 and 4.163grams of H2O. (a) Calculate the masses of C and H in the sample. Grams: C= g; H= 9 (b) Does the compound contain any other elements? (c) What are the mass percentages of C and H in the compound? Mass percentages: C= %;H= % (d) What is the empirical formula of the compound? Enter the elements in the order presented in the question. empirical formula = 9 more group attempte romaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


