Question: Use the References to access important values if needed for this question. The nonvolatile, nonelectrolyte urea, CH4N2O(60.1g/mol), is soluble in water, H2O. How many grams
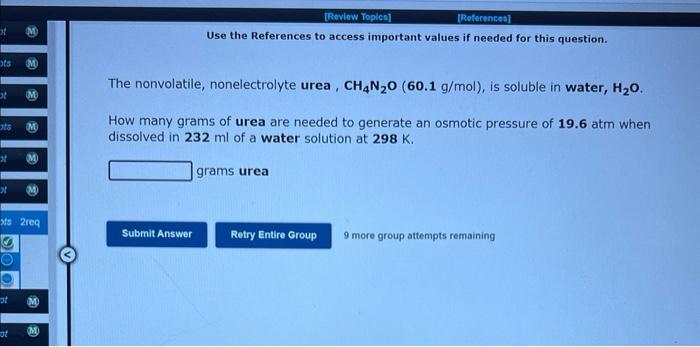

Use the References to access important values if needed for this question. The nonvolatile, nonelectrolyte urea, CH4N2O(60.1g/mol), is soluble in water, H2O. How many grams of urea are needed to generate an osmotic pressure of 19.6atm when dissolved in 232ml of a water solution at 298K. grams urea 9 more group attempts remaining Use the References to access important values if needed for this question. Match the following aqueous solutions with the appropriate letter from the column on the right. 1. 0.16mCoI2 A. Lowest freezing point 2. 0.14m(NH4)3PO4 B. Second lowest freezing point 3. 0.17mMgCl2 C. Third lowest freezing point 4. 0.54 m Urea(nonelectrolyte) D. Highest freezing point 3 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


