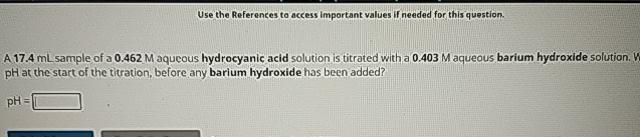
Question: Use the References to access important values if needed for this question. A 1 7 . 4 m L sample of a 0 . 4
Use the References to access important values if needed for this question.
A sample of a aqueous hydrocyanic acid solution is titrated with a aqueous barium hydroxide solution. at the start of the titration, before any barium hydroxide has been added?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


