Question: Use the References to access important values if needed for this question. The equilibrium constant, Kc, for the following reaction is 55.6 at 698 K.
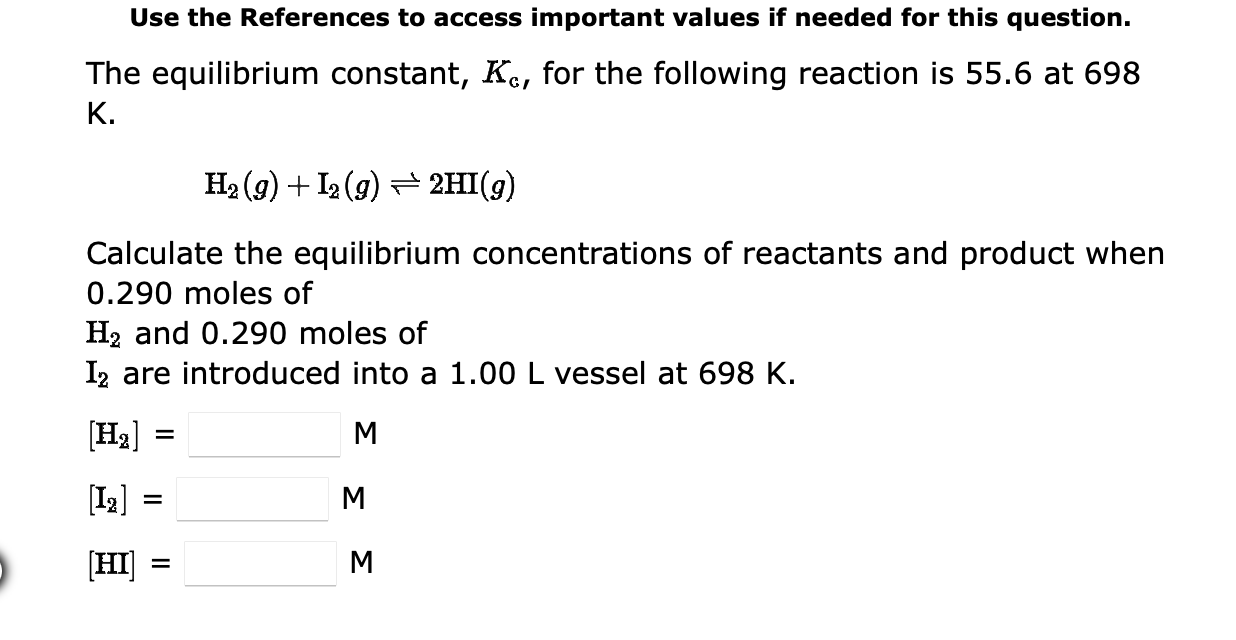
Use the References to access important values if needed for this question. The equilibrium constant, Kc, for the following reaction is 55.6 at 698 K. H2(g)+I2(g)2HI(g) Calculate the equilibrium concentrations of reactants and product when 0.290 moles of H2 and 0.290 moles of I2 are introduced into a 1.00L vessel at 698K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


