Question: Using Henry's Law constants in the table given below, calculate the solubility in M of each gas in water at 25C if TABLE 6.1 Henry's
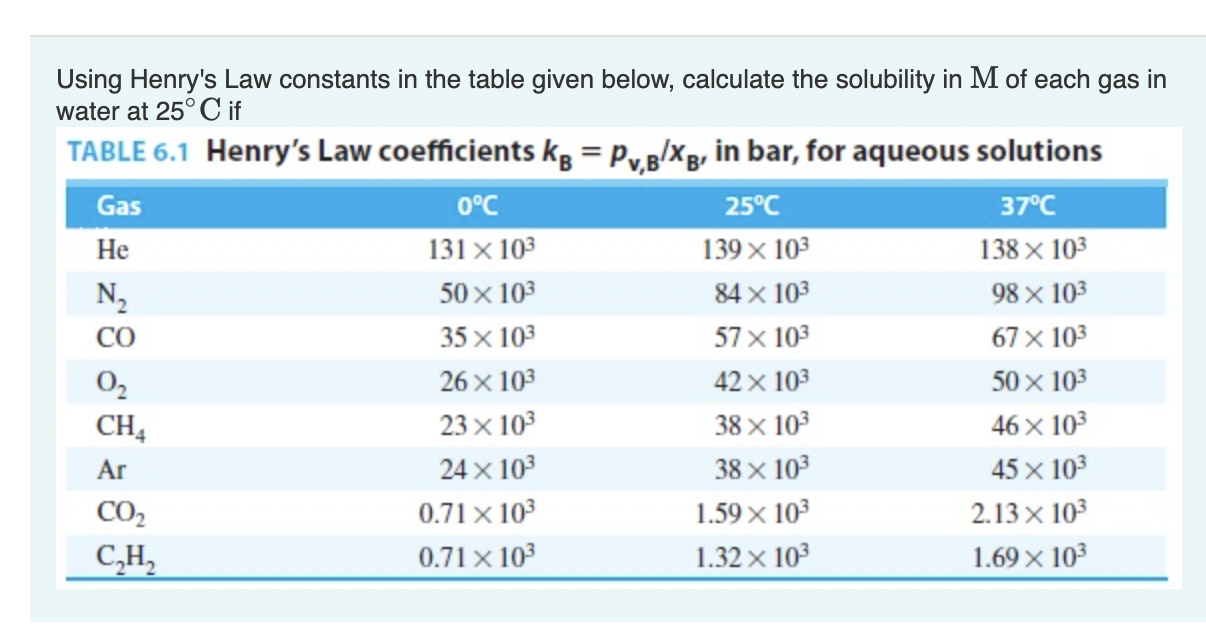
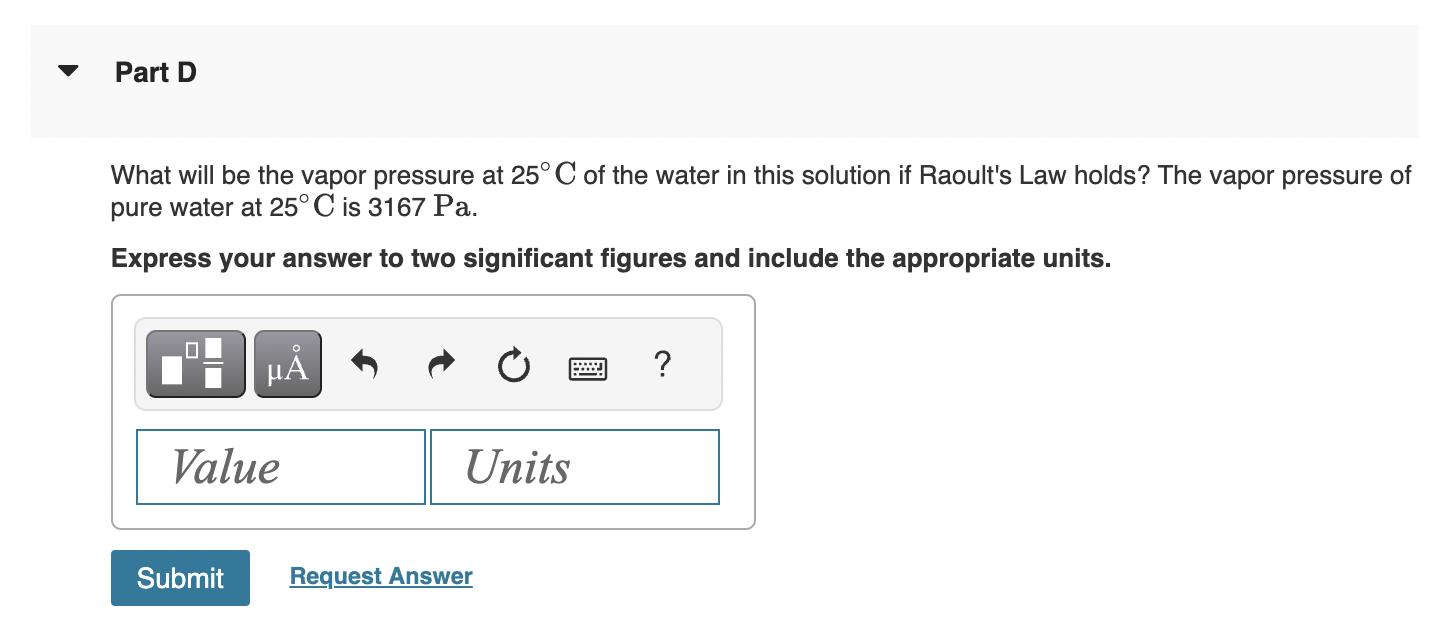
Using Henry's Law constants in the table given below, calculate the solubility in M of each gas in water at 25C if TABLE 6.1 Henry's Law coefficients kB=pv,B/xB in bar, for aqueous solutions What will be the vapor pressure at 25C of the water in this solution if Raoult's Law holds? The vapor pressure of pure water at 25C is 3167Pa. Express your answer to two significant figures and include the appropriate units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


