Question: Using the bond energy data below, determine the change in energy (in kJ) that occurs for the ifollowing reaction acetylene +2 hydrogen ethane acetylene has
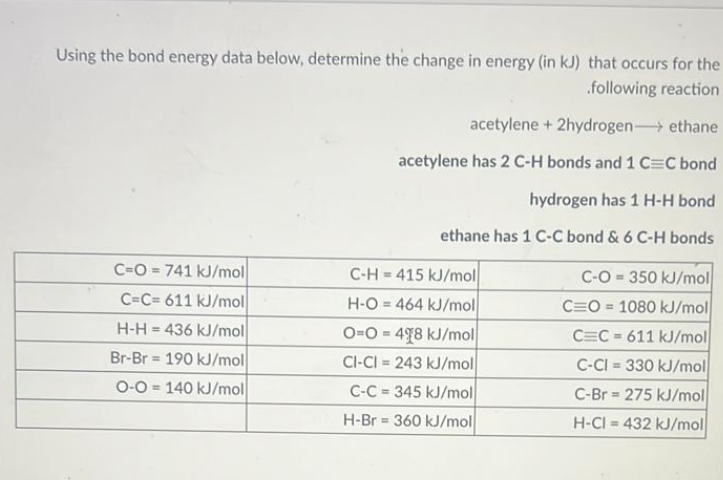
Using the bond energy data below, determine the change in energy (in kJ) that occurs for the ifollowing reaction acetylene +2 hydrogen ethane acetylene has 2CH bonds and 1CC bond hydrogen has 1HH bond ethane has 1CC bond &6CH bonds
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


