Question: Using the following standard electrode potentials to calculate the Ecell (V) for the: Fe2+(aq) + 2e- Fe (s) E =-0.44 V Zn2+(aq) + 2e Zn
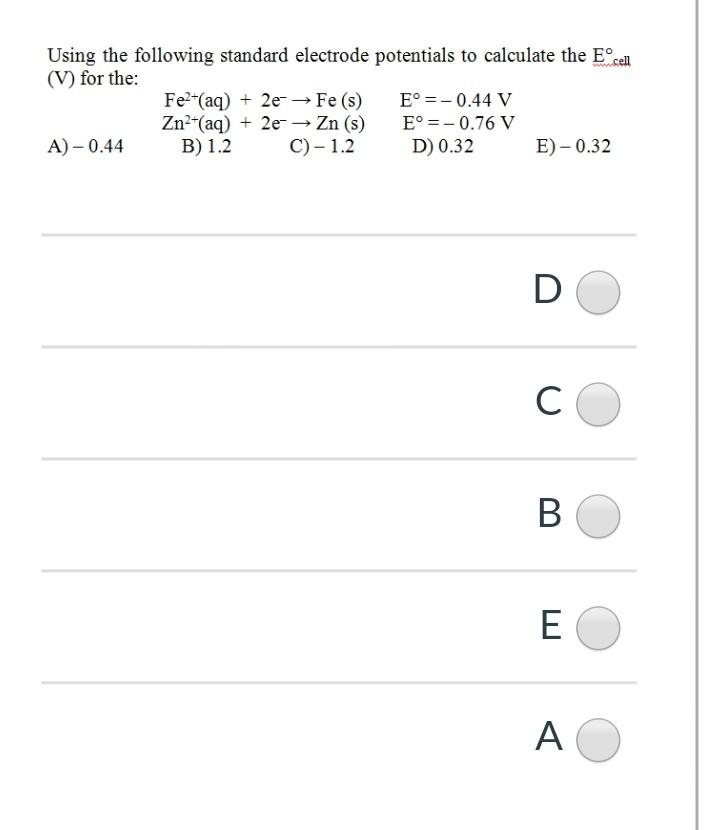
Using the following standard electrode potentials to calculate the Ecell (V) for the: Fe2+(aq) + 2e- Fe (s) E =-0.44 V Zn2+(aq) + 2e Zn (s) E = -0.76 V A)-0.44 B) 1.2 C) - 1.2 D) 0.32 E-0.32 D O CO E ( O
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


