Question: Werence Use the References to access important values if needed for this question In a study of the decomposition of ammonia on a tungsten surface
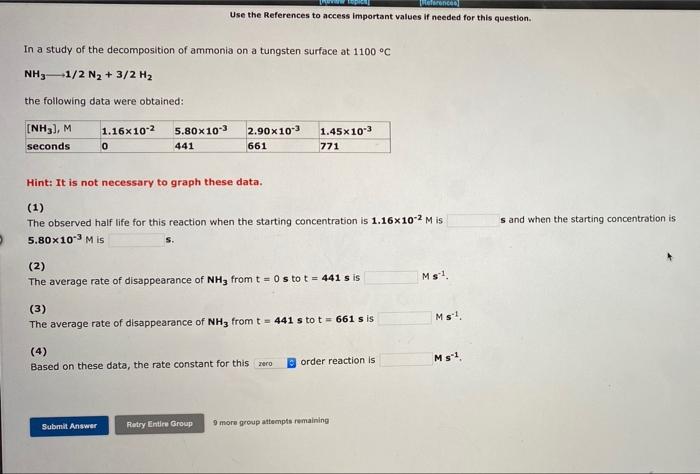
Werence Use the References to access important values if needed for this question In a study of the decomposition of ammonia on a tungsten surface at 1100 C NH3-1/2 N2 + 3/2 H2 the following data were obtained: (NH3), M seconds 1.16x10-2 0 5.80X10-3 441 2.90 x 10" 661 1.45x10-3 771 Hint: It is not necessary to graph these data. (1) The observed half life for this reaction when the starting concentration is 1.16x10-2 Mis 5.80 x 10-3 M is s and when the starting concentration is Ms1 (2) The average rate of disappearance of NHfrom t = 0 s to t = 441 s is (3) The average rate of disappearance of NH3 from t = 441 s to t = 661 s is (4) Based on these data, the rate constant for this zero Ms! order reaction is Ms1 Submit Answer Ratry Entire Group 9 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


