Question: What is, depending on the temperature, the partial oxygen pressure in equilibrium with a CuO - Cu20 mixture? Also do the calculation for 500 and
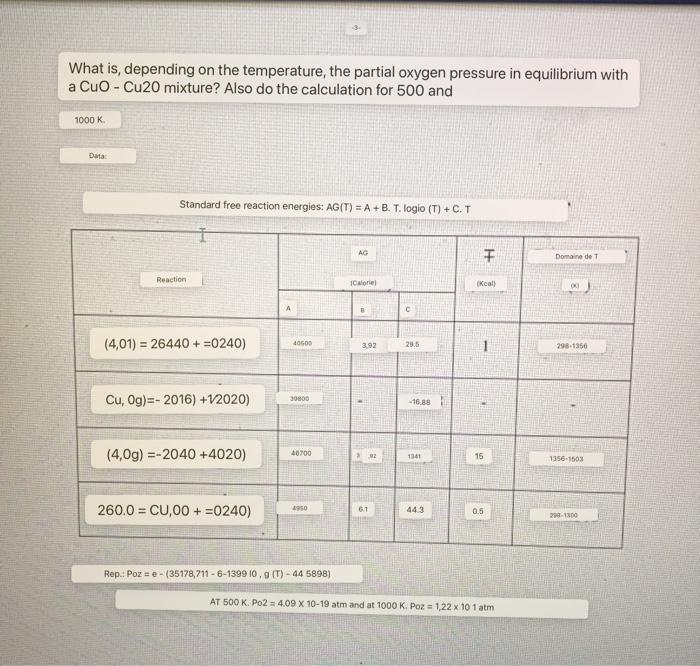
What is, depending on the temperature, the partial oxygen pressure in equilibrium with a CuO - Cu20 mixture? Also do the calculation for 500 and Standard free reaction energies: AG(T)=A+B.T. logio (T)+C.T Rep: Poz =e(35178,7116139910,g(T)445898)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


